Transporters MRP1 and MRP2 Regulate Opposing Inflammatory Signals To Control Transepithelial Neutrophil Migration during Streptococcus pneumoniae Lung Infection
- PMID: 29976647
- PMCID: PMC6034076
- DOI: 10.1128/mSphere.00303-18
Transporters MRP1 and MRP2 Regulate Opposing Inflammatory Signals To Control Transepithelial Neutrophil Migration during Streptococcus pneumoniae Lung Infection
Abstract
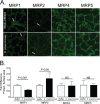
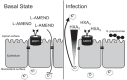
Streptococcus pneumoniae remains a source of morbidity and mortality in both developed and underdeveloped nations of the world. Disease can manifest as pneumonia, bacteremia, and meningitis, depending on the localization of infection. Interestingly, there is a correlation in experimental murine infections between the development of bacteremia and influx of neutrophils into the pulmonary lumen. Reduction of this neutrophil influx has been shown to improve survivability during infection. In this study, we use in vitro biotinylation and neutrophil transmigration and in vivo murine infection to identify a system in which two epithelium-localized ATP-binding cassette transporters, MRP1 and MRP2, have inverse activities dictating neutrophil transmigration into the lumen of infected mouse lungs. MRP1 effluxes an anti-inflammatory molecule that maintains homeostasis in uninfected contexts, thus reducing neutrophil infiltration. During inflammatory events, however, MRP1 decreases and MRP2 both increases and effluxes the proinflammatory eicosanoid hepoxilin A3. If we then decrease MRP2 activity during experimental murine infection with S. pneumoniae, we reduce both neutrophil infiltration and bacteremia, showing that MRP2 coordinates this activity in the lung. We conclude that MRP1 assists in depression of polymorphonuclear cell (PMN) migration by effluxing a molecule that inhibits the proinflammatory effects of MRP2 activity.IMPORTANCEStreptococcus pneumoniae is a Gram-positive bacterium that normally inhabits the human nasopharynx asymptomatically. However, it is also a major cause of pneumonia, bacteremia, and meningitis. The transition from pneumonia to bacteremia is critical, as patients that develop septicemia have ~20% mortality rates. Previous studies have shown that while neutrophils, a major bacterium-induced leukocyte, aid in S. pneumoniae elimination, they also contribute to pathology and may mediate the lung-to-blood passage of the bacteria. Herein, we show that epithelium-derived MRP1 and MRP2 efflux immunomodulatory agents that assist in controlling passage of neutrophils during infection and that limiting neutrophil infiltration produced less bacteremia and better survival during murine infection. The importance of our work is twofold: ours is the first to identify an MRP1/MRP2 axis of neutrophil control in the lung. The second is to provide possible therapeutic targets to reduce excess inflammation, thus reducing the chances of developing bacteremia during pneumococcal pneumonia.
Keywords: MRP1; MRP2; PMN; Streptococcus pneumoniae; hepoxilin A3; neutrophil; pneumococcus.
Copyright © 2018 Zukauskas et al.
Figures




Similar articles
-
Blocking HXA3-mediated neutrophil elastase release during S. pneumoniae lung infection limits pulmonary epithelial barrier disruption and bacteremia.mBio. 2024 Sep 11;15(9):e0185624. doi: 10.1128/mbio.01856-24. Epub 2024 Aug 9. mBio. 2024. PMID: 39120139 Free PMC article.
-
Systemic disease during Streptococcus pneumoniae acute lung infection requires 12-lipoxygenase-dependent inflammation.J Immunol. 2013 Nov 15;191(10):5115-23. doi: 10.4049/jimmunol.1300522. Epub 2013 Oct 2. J Immunol. 2013. PMID: 24089193 Free PMC article.
-
The α-tocopherol form of vitamin E reverses age-associated susceptibility to streptococcus pneumoniae lung infection by modulating pulmonary neutrophil recruitment.J Immunol. 2015 Feb 1;194(3):1090-9. doi: 10.4049/jimmunol.1402401. Epub 2014 Dec 15. J Immunol. 2015. PMID: 25512603 Free PMC article.
-
Neutrophil Recruitment in Pneumococcal Pneumonia.Front Cell Infect Microbiol. 2022 May 13;12:894644. doi: 10.3389/fcimb.2022.894644. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35646729 Free PMC article. Review.
-
The Role of Neutrophils and Neutrophil Elastase in Pneumococcal Pneumonia.Front Cell Infect Microbiol. 2021 Mar 16;11:615959. doi: 10.3389/fcimb.2021.615959. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33796475 Free PMC article. Review.
Cited by
-
A Six-gene Prognostic Model Based on Neutrophil Extracellular Traps (NETs)-related Gene Signature for Lung Adenocarcinoma.Comb Chem High Throughput Screen. 2024;27(13):1969-1983. doi: 10.2174/0113862073282003240119064337. Comb Chem High Throughput Screen. 2024. PMID: 38357943
-
miRNA-302e attenuates inflammation in infantile pneumonia though the RelA/BRD4/NF-κB signaling pathway.Int J Mol Med. 2019 Jul;44(1):47-56. doi: 10.3892/ijmm.2019.4194. Epub 2019 May 10. Int J Mol Med. 2019. PMID: 31115487 Free PMC article.
-
Development of ADS051, an oral, gut-restricted, small molecule neutrophil modulator for the treatment of neutrophil-mediated inflammatory diseases.FEBS Open Bio. 2023 Aug;13(8):1434-1446. doi: 10.1002/2211-5463.13668. Epub 2023 Jul 10. FEBS Open Bio. 2023. PMID: 37392453 Free PMC article.
-
The Yin and Yang of Pneumolysin During Pneumococcal Infection.Front Immunol. 2022 Apr 22;13:878244. doi: 10.3389/fimmu.2022.878244. eCollection 2022. Front Immunol. 2022. PMID: 35529870 Free PMC article. Review.
References
-
- Centers for Disease Control and Prevention 2015. Epidemiology and prevention of vaccine-preventable diseases, 13th ed. Communication and Education Branch, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, GA.
-
- Centers for Disease Control and Prevention 2012. Manual for the surveillance of vaccine-preventable diseases. National Center for Immunization and Respiratory Disease, Centers for Disease Control and Prevention, Atlanta, GA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
