Targeting the HIV-1 Spike and Coreceptor with Bi- and Trispecific Antibodies for Single-Component Broad Inhibition of Entry
- PMID: 29976677
- PMCID: PMC6146690
- DOI: 10.1128/JVI.00384-18
Targeting the HIV-1 Spike and Coreceptor with Bi- and Trispecific Antibodies for Single-Component Broad Inhibition of Entry
Erratum in
-
Correction for Khan et al., "Targeting the HIV-1 Spike and Coreceptor with Bi- and Trispecific Antibodies for Single-Component Broad Inhibition of Entry".J Virol. 2019 May 15;93(11):e00391-19. doi: 10.1128/JVI.00391-19. Print 2019 Jun 1. J Virol. 2019. PMID: 31092683 Free PMC article. No abstract available.
Abstract
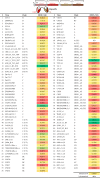
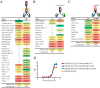
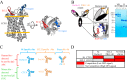
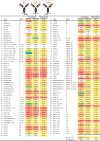
Protection against acquiring human immunodeficiency virus (HIV) infection may not require a vaccine in the conventional sense, because broadly neutralizing antibodies (bNAbs) alone prevent HIV infection in relevant animal challenge models. Additionally, bNAbs as therapeutics can effectively suppress HIV replication in infected humans and in animal models. Combinations of bNAbs are generally even more effective, and bNAb-derived multivalent antibody-like molecules also inhibit HIV replication both in vitro and in vivo To expand the available array of multispecific HIV inhibitors, we designed single-component molecules that incorporate two (bispecific) or three (trispecific) bNAbs that recognize HIV Env exclusively, a bispecific CrossMAb targeting two epitopes on the major HIV coreceptor, CCR5, and bi- and trispecifics that cross-target both Env and CCR5. These newly designed molecules displayed exceptional breadth, neutralizing 98 to 100% of a 109-virus panel, as well as additivity and potency compared to those of the individual parental control IgGs. The bispecific molecules, designed as tandem single-chain variable fragments (scFvs) (10E8fv-N6fv and m36.4-PRO 140fv), displayed median 50% inhibitory concentration (IC50s) of 0.0685 and 0.0131 μg/ml, respectively. A trispecific containing 10E8-PGT121-PGDM1400 Env-specific binding sites was equally potent (median IC50 of 0.0135 μg/ml), while a trispecific molecule targeting Env and CCR5 simultaneously (10E8Fab-PGDM1400fv-PRO 140fv) demonstrated even greater potency, with a median IC50 of 0.007 μg/ml. By design, some of these molecules lacked Fc-mediated effector function; therefore, we also constructed a trispecific prototype possessing reconstituted CH2-CH3 domains to restore Fc receptor binding capacity. The molecules developed here, along with those described previously, possess promise as prophylactic and therapeutic agents against HIV.IMPORTANCE Broadly neutralizing antibodies (bNAbs) prevent HIV infection in monkey challenge models and suppress HIV replication in infected humans. Combinations of bNAbs are more effective at suppression, and antibody-like molecules engineered to have two or three bNAb combining sites also inhibit HIV replication in monkeys and other animal models. To expand the available array of multispecific HIV inhibitors, we designed single-component molecules that incorporate two (bispecific) or three (trispecific) bNAb binding sites that recognize the HIV envelope glycoprotein (Env) or the HIV coreceptor (CCR5) or that cross-target both Env and CCR5. Several of the bi- and trispecific molecules neutralized most viruses in a diverse cross-clade panel, with greater breadth and potency than those of the individual parental bNAbs. The molecules described here provide additional options for preventing or suppressing HIV infection.
Keywords: CrossMAb; HIV envelope; HIV-1; anti-CCR5; bispecific antibody; trispecific antibody.
Copyright © 2018 American Society for Microbiology.
Figures





References
-
- Sok D, Le KM, Vadnais M, Saye-Francisco KL, Jardine JG, Torres JL, Berndsen ZT, Kong L, Stanfield R, Ruiz J, Ramos A, Liang CH, Chen PL, Criscitiello MF, Mwangi W, Wilson IA, Ward AB, Smider VV, Burton DR. 2017. Rapid elicitation of broadly neutralizing antibodies to HIV by immunization in cows. Nature 548:108–111. doi: 10.1038/nature23301. - DOI - PMC - PubMed
-
- Banerjee K, Klasse PJ, Sanders RW, Pereyra F, Michael E, Lu M, Walker BD, Moore JP. 2010. IgG subclass profiles in infected HIV type 1 controllers and chronic progressors and in uninfected recipients of Env vaccines. AIDS Res Hum Retroviruses 26:445–458. doi: 10.1089/aid.2009.0223. - DOI - PMC - PubMed
-
- Huang J, Kang BH, Ishida E, Zhou T, Griesman T, Sheng Z, Wu F, Doria-Rose NA, Zhang B, McKee K, O'Dell S, Chuang GY, Druz A, Georgiev IS, Schramm CA, Zheng A, Joyce MG, Asokan M, Ransier A, Darko S, Migueles SA, Bailer RT, Louder MK, Alam SM, Parks R, Kelsoe G, Von Holle T, Haynes BF, Douek DC, Hirsch V, Seaman MS, Shapiro L, Mascola JR, Kwong PD, Connors M. 2016. Identification of a CD4-binding-site antibody to HIV that evolved near-pan neutralization breadth. Immunity 45:1108–1121. doi: 10.1016/j.immuni.2016.10.027. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

