Preclinical human models and emerging therapeutics for advanced systemic mastocytosis
- PMID: 29976735
- PMCID: PMC6278969
- DOI: 10.3324/haematol.2018.195867
Preclinical human models and emerging therapeutics for advanced systemic mastocytosis
Abstract
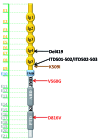
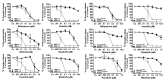
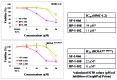
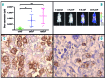
Mastocytosis is a term used to denote a group of rare diseases characterized by an abnormal accumulation of neoplastic mast cells in various tissues and organs. In most patients with systemic mastocytosis, the neoplastic cells carry activating mutations in KIT Progress in mastocytosis research has long been hindered by the lack of suitable in vitro models, such as permanent human mast cell lines. In fact, only a few human mast cell lines are available to date: HMC-1, LAD1/2, LUVA, ROSA and MCPV-1. The HMC-1 and LAD1/2 cell lines were derived from patients with mast cell leukemia. By contrast, the more recently established LUVA, ROSA and MCPV-1 cell lines were derived from CD34+ cells of non-mastocytosis donors. While some of these cell lines (LAD1/2, LUVA, ROSAKIT WT and MCPV-1) do not harbor KIT mutations, HMC-1 and ROSAKIT D816V cells exhibit activating KIT mutations found in mastocytosis and have thus been used to study disease pathogenesis. In addition, these cell lines are increasingly employed to validate new therapeutic targets and to screen for effects of new targeted drugs. Recently, the ROSAKIT D816V subclone has been successfully used to generate a unique in vivo model of advanced mastocytosis by injection into immunocompromised mice. Such a model may allow in vivo validation of data obtained in vitro with targeted drugs directed against mastocytosis. In this review, we discuss the major characteristics of all available human mast cell lines, with particular emphasis on the use of HMC-1 and ROSAKIT D816V cells in preclinical therapeutic research in mastocytosis.
Copyright© 2018 Ferrata Storti Foundation.
Figures
References
-
- DeBruin EJ, Gold M, Lo BC, et al. Mast cells in human health and disease. Methods Mol Biol. 2015;1220:93–119. - PubMed
-
- Kirshenbaum AS, Metcalfe DD. Growth of human mast cells from bone marrow and peripheral blood-derived CD34+ pluripotent progenitor cells. Methods Mol Biol. 2006;315:105–112. - PubMed
-
- Galli SJ, Tsai M, Wershil BK, Tam SY, Costa JJ. Regulation of mouse and human mast cell development, survival and function by stem cell factor, the ligand for the c-kit receptor. Int Arch Allergy Immunol. 1995;107(1–3):51–53. - PubMed
-
- Brown JM, Wilson TM, Metcalfe DD. The mast cell and allergic diseases: role in pathogenesis and implications for therapy. Clin Exp Allergy. 2008;38(1):4–18. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

