Serum Amyloid A Is an Exchangeable Apolipoprotein
- PMID: 29976766
- PMCID: PMC6202200
- DOI: 10.1161/ATVBAHA.118.310979
Serum Amyloid A Is an Exchangeable Apolipoprotein
Abstract
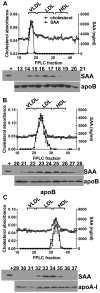
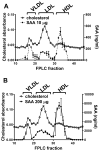
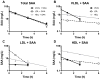
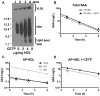
Objective- SAA (serum amyloid A) is a family of acute-phase reactants that have proinflammatory and proatherogenic activities. SAA is more lipophilic than apoA-I (apolipoprotein A-I), and during an acute-phase response, <10% of plasma SAA is found lipid-free. In most reports, SAA is found exclusively associated with high-density lipoprotein; however, we and others have reported SAA on apoB (apolipoprotein B)-containing lipoproteins in both mice and humans. The goal of this study was to determine whether SAA is an exchangeable apolipoprotein. Approach and Results- Delipidated human SAA was incubated with SAA-free human lipoproteins; then, samples were reisolated by fast protein liquid chromatography, and SAA analyzed by ELISA and immunoblot. Both in vitro and in vivo, we show that SAA associates with any lipoprotein and does not remain in a lipid-free form. Although SAA is preferentially found on high-density lipoprotein, it can exchange between lipoproteins. In the presence of CETP (cholesterol ester transfer protein), there is greater exchange of SAA between lipoproteins. Subjects with diabetes mellitus, but not those with metabolic syndrome, showed altered SAA lipoprotein distribution postprandially. Proteoglycan-mediated lipoprotein retention is thought to be an underlying mechanism for atherosclerosis development. SAA has a proteoglycan-binding domain. Lipoproteins containing SAA had increased proteoglycan binding compared with SAA-free lipoproteins. Conclusions- Thus, SAA is an exchangeable apolipoprotein and increases apoB-containing lipoproteins' proteoglycan binding. We and others have previously reported the presence of SAA on low-density lipoprotein in individuals with obesity, diabetes mellitus, and metabolic syndrome. We propose that the presence of SAA on apoB-containing lipoproteins may contribute to cardiovascular disease development in these populations.
Keywords: apolipoproteins; atherosclerosis; inflammation; metabolic syndrome; models, animal.
Figures


References
-
- Kisilevsky R, Manley PN. Acute-phase serum amyloid a: Perspectives on its physiological and pathological roles. Amyloid. 2012;19:5–14. - PubMed
-
- Johnson BD, Kip KE, Marroquin OC, Ridker PM, Kelsey SF, Shaw LJ, Pepine CJ, Sharaf B, Bairey Merz CN, Sopko G, Olson MB, Reis SE. Serum amyloid a as a predictor of coronary artery disease and cardiovascular outcome in women: The national heart, lung, and blood institute-sponsored women’s ischemia syndrome evaluation (wise) Circulation. 2004;109:726–732. - PubMed
-
- Kosuge M, Ebina T, Ishikawa T, Hibi K, Tsukahara K, Okuda J, Iwahashi N, Ozaki H, Yano H, Kusama I, Nakati T, Umemura S, Kimura K. Serum amyloid a is a better predictor of clinical outcomes than c-reactive protein in non-st-segment elevation acute coronary syndromes. Circ J. 2007;71:186–190. - PubMed
-
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

