Induced pluripotent stem cell-derived endothelial cells promote angiogenesis and accelerate wound closure in a murine excisional wound healing model
- PMID: 29976773
- PMCID: PMC6066657
- DOI: 10.1042/BSR20180563
Induced pluripotent stem cell-derived endothelial cells promote angiogenesis and accelerate wound closure in a murine excisional wound healing model
Abstract
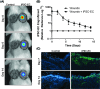
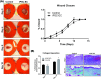
Chronic wounds are a major complication in patients with cardiovascular diseases. Cell therapies have shown potential to stimulate wound healing, but clinical trials using adult stem cells have been tempered by limited numbers of cells and invasive procurement procedures. Induced pluripotent stem cells (iPSCs) have several advantages of other cell types, for example they can be generated in abundance from patients' somatic cells (autologous) or those from a matched donor. iPSCs can be efficiently differentiated to functional endothelial cells (iPSC-ECs). Here, we used a murine excisional wound model to test the pro-angiogenic properties of iPSC-ECs in wound healing. Two full-thickness wounds were made on the dorsum of NOD-SCID mice and splinted. iPSC-ECs (5 × 105) were topically applied to one wound, with the other serving as a control. Treatment with iPSC-ECs significantly increased wound perfusion and accelerated wound closure. Expression of endothelial cell (EC) surface marker, platelet endothelial cell adhesion molecule (PECAM-1) (CD31), and pro-angiogenic EC receptor, Tie1, mRNA was up-regulated in iPSC-EC treated wounds at 7 days post-wounding. Histological analysis of wound sections showed increased capillary density in iPSC-EC wounds at days 7 and 14 post-wounding, and increased collagen content at day 14. Anti-GFP fluorescence confirmed presence of iPSC-ECs in the wounds. Bioluminescent imaging (BLI) showed progressive decline of iPSC-ECs over time, suggesting that iPSC-ECs are acting primarily through short-term paracrine effects. These results highlight the pro-regenerative effects of iPSC-ECs and demonstrate that they are a promising potential therapy for intractable wounds.
Keywords: angiogenesis; endothelial cells; induced pluripotent stem cells; wound healing.
© 2018 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures



References
-
- Leavitt T., Hu M.S., Marshall C.D., Barnes L.A., Longaker M.T. and Lorenz H.P. (2016) Stem cells and chronic wound healing: state of the art. Chronic Wound Care Management Res. 3, 7–27
-
- Hinchliffe R.J., Brownrigg J.R.W., Andros G., Apelqvist J., Boyko E.J., Fitridge R. et al. (2016) Effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral artery disease: a systematic review. Diabetes Metab. Res. Rev. 32, 136–144 10.1002/dmrr.2705 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

