Adherence and long-term growth outcomes: results from the easypod™ connect observational study (ECOS) in paediatric patients with growth disorders
- PMID: 29976785
- PMCID: PMC6107763
- DOI: 10.1530/EC-18-0172
Adherence and long-term growth outcomes: results from the easypod™ connect observational study (ECOS) in paediatric patients with growth disorders
Abstract
Objective: The easypod connect observational study (ECOS) assessed treatment adherence among paediatric patients receiving growth hormone (GH) via the easypod electronic injection device.
Design: ECOS was an open-label, observational, longitudinal study conducted in 24 countries between 2010 and 2016, enrolling children treated with GH.
Methods: The primary endpoint was the rate of treatment adherence during 5 years of follow-up. Impact of adherence on growth outcomes was assessed using Spearman's product-moment correlations.
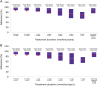
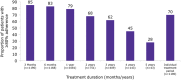
Results and conclusions: Overall, 1190 patients had easypod data available for ≥3 months; most patients had GH deficiency (75%); 606 of these patients were GH naïve at baseline. Over the first year of monitoring, the median rate of adherence was 93.7% among patients overall and >93.0% in GH-naïve patients, irrespective of the treatment indication. Clinically meaningful improvements in growth rates were observed after 1 year of treatment across all GH indications. Adherence decreased with increasing treatment duration, but the overall median adherence rate remained high after 3 years of follow-up: 87.2% (n = 409), 75.5% after 4 years (n = 143) and 70.2% after 5 years (n = 43). Statistically significant correlations between adherence and 1-year change in height standard deviation score (P < 0.001 for patients overall) and height velocity (P < 0.001) were observed.
Conclusions: ECOS produced accurate, real-time adherence data in a large population of GH-treated children over 5 years of follow-up. Using the easypod connect system, physicians can potentially identify patients with inadequate adherence and poor response to treatment, enabling them to take appropriate action to help them maximise the benefits of GH treatment.
Keywords: GH treatment; adherence; e-Health; easypod.
© 2018 The authors.
Figures
References
-
- Takeda A, Cooper K, Bird A, Baxter L, Frampton GK, Gospodarevskaya E, Welch K, Bryant J. Recombinant human growth hormone for the treatment of growth disorders in children: a systematic review and economic evaluation. Health Technology Assessment 2010. 14 1–209, iii–iv. ( 10.3310/hta14420) - DOI - PubMed
-
- Grimberg A, DiVall SA, Polychronakos C, Allen DB, Cohen LE, Quintos JB, Rossi WC, Feudtner C, Murad MH. Guidelines for growth hormone and insulin-like growth factor-I treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Hormone Research in Paediatrics 2016. 86 361–397. ( 10.1159/000452150) - DOI - PubMed
-
- Richmond E, Rogol AD. Current indications for growth hormone therapy for children and adolescents. Endocrine Development 2010. 18 92–108. - PubMed
-
- Ranke M, Lindberg A, Cowell C, Wikland K, Reiter E, Wilton P, Price D. Prediction of response to growth hormone treatment in short children born small for gestational age: analysis of data from KIGS (Pharmacia International Growth Database). Journal of Clinical Endocrinology and Metabolism 2003. 88 125–131. ( 10.1210/jc.2002-020867) - DOI - PubMed
-
- Sas TC, de Ridder MA, Wit JM, Rotteveel J, Oostdijk W, Reeser HM, Otten BJ, de Muinck Keizer-Schrama SM. Adult height in children with growth hormone deficiency: a randomized, controlled, growth hormone dose-response trial. Hormone Research in Paediatrics 2010. 74 172–181. ( 10.1159/000281323) - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

