Zika Virus Replication in Dorsal Root Ganglia Explants from Interferon Receptor1 Knockout Mice Causes Myelin Degeneration
- PMID: 29976926
- PMCID: PMC6033858
- DOI: 10.1038/s41598-018-28257-5
Zika Virus Replication in Dorsal Root Ganglia Explants from Interferon Receptor1 Knockout Mice Causes Myelin Degeneration
Abstract
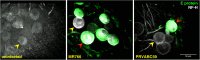
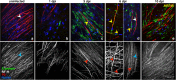
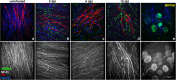
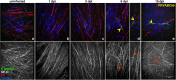
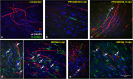
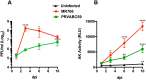
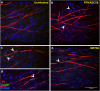
Zika virus (ZIKV) is a neurotropic agent that targets the developing fetal brain in women infected during pregnancy. In addition to the developing central nervous system, ZIKV has been recently shown to infect cells of the peripheral nervous system (PNS), highlighting its potential to cause acute peripheral neuropathies in adults, such as Guillain-Barré Syndrome (GBS). Here we show that myelinating dorsal root ganglia (DRG) explants obtained from interferon-alpha/beta receptor knock-out mice are productively infected by ZIKV. Virus replication is cytopathic in both peripheral neurons and myelinating Schwann cells leading to myelin disruption. These results confirm and extend previous observations suggesting that the PNS is indeed a potential site of ZIKV infection, replication and cytopathicity.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

