Modeling erythrocyte electrodeformation in response to amplitude modulated electric waveforms
- PMID: 29976935
- PMCID: PMC6033869
- DOI: 10.1038/s41598-018-28503-w
Modeling erythrocyte electrodeformation in response to amplitude modulated electric waveforms
Abstract
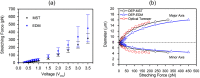
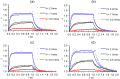
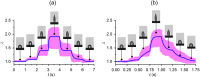
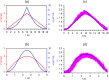
We present a comprehensive theoretical-experimental framework for quantitative, high-throughput study of cell biomechanics. An improved electrodeformation method has been developed by combing dielectrophoresis and amplitude shift keying, a form of amplitude modulation. This method offers a potential to fully control the magnitude and rate of deformation in cell membranes. In healthy human red blood cells, nonlinear viscoelasticity of cell membranes is obtained through variable amplitude load testing. A mathematical model to predict cellular deformations is validated using the experimental results of healthy human red blood cells subjected to various types of loading. These results demonstrate new capabilities of the electrodeformation technique and the validated mathematical model to explore the effects of different loading configurations on the cellular mechanical behavior. This gives it more advantages over existing methods and can be further developed to study the effects of strain rate and loading waveform on the mechanical properties of biological cells in health and disease.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Salehyar, S. & Zhu, Q. Effects of stiffness and volume on the transit time of an erythrocyte through a slit. Biomechanics and modeling in mechanobiology, 1–11 (2017). - PubMed
-
- Riva CE, Grunwald JE, Sinclair SH, Petrig B. Blood velocity and volumetric flow rate in human retinal vessels. Investigative ophthalmology & visual science. 1985;26:1124–1132. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

