Developing a conceptual, reproducible, rubric-based approach to consent and result disclosure for genetic testing by clinicians with minimal genetics background
- PMID: 29976988
- PMCID: PMC6320736
- DOI: 10.1038/s41436-018-0093-6
Developing a conceptual, reproducible, rubric-based approach to consent and result disclosure for genetic testing by clinicians with minimal genetics background
Abstract
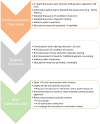
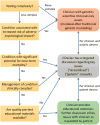
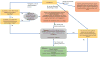
Purpose: In response to genetic testing being widely ordered by nongenetics clinicians, the Consent and Disclosure Recommendations (CADRe) Workgroup of the Clinical Genome Resource (ClinGen; clinicalgenome.org ) developed guidance to facilitate communication about genetic testing and efficiently improve the patient experience. Considering ethical, legal, and social implications, and medical factors, CADRe developed and pilot tested two rubrics addressing consent for genetic testing and results disclosure. The CADRe rubrics allow for adjusting the communication approach based on circumstances specific to patients and ordering clinicians.
Methods: We present results of a formative survey of 66 genetics clinicians to assess the consent rubric for nine genes (MLH1, CDH1, TP53, GJB2, OTC; DMD, HTT, and CYP2C9/VKORC1). We also conducted interviews and focus groups with family and patient stakeholders (N = 18), nongenetics specialists (N = 27), and genetics clinicians (N = 32) on both rubrics.
Results: Formative evaluation of the CADRe rubrics suggests key factors on which to make decisions about consent and disclosure discussions for a "typical" patient.
Conclusion: We propose that the CADRe rubrics include the primary issues necessary to guide communication recommendations, and are ready for pilot testing by nongenetics clinicians. Consultation with genetics clinicians can be targeted toward more complex or intensive consent and disclosure counseling.
Keywords: Genetic counseling,; Genetic testing,; Informed consent; Results disclosure; Rubric.
Conflict of interest statement
Seema Jamal is an employee at GeneDx. Misha Raskin is an employee and stockholder at Helix, LLC.
Figures
References
-
- Chambers CV, Axell-House DB, Mills G, et al. Primary care physicians’ experience and confidence with genetic testing and perceived barriers to genomic medicine. J Fam Med. 2015;2(2):1024.
-
- Bensend TA, Veach PM, Niendorf KB. What’s the harm? Genetic counselor perceptions of adverse effects of genetics service provision by non-genetics professionals. J Genet Couns. 2014;23:48–63. - PubMed
-
- Brierley KL, Blouch E, Cogswell W, et al. Adverse events in cancer genetic testing: medical, ethical, legal, and financial implications. Cancer J. 2012;18:303–309. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

