Blue light excited retinal intercepts cellular signaling
- PMID: 29976989
- PMCID: PMC6033873
- DOI: 10.1038/s41598-018-28254-8
Blue light excited retinal intercepts cellular signaling
Abstract
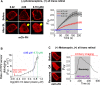
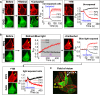
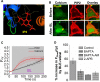
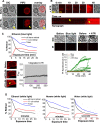
Photoreceptor chromophore, 11-cis retinal (11CR) and the photoproduct, all-trans retinal (ATR), are present in the retina at higher concentrations and interact with the visual cells. Non-visual cells in the body are also exposed to retinal that enters the circulation. Although the cornea and the lens of the eye are transparent to the blue light region where retinal can absorb and undergo excitation, the reported phototoxicity in the eye has been assigned to lipophilic non-degradable materials known as lipofuscins, which also includes retinal condensation products. The possibility of blue light excited retinal interacting with cells; intercepting signaling in the presence or absence of light has not been explored. Using live cell imaging and optogenetic signaling control, we uncovered that blue light-excited ATR and 11CR irreversibly change/distort plasma membrane (PM) bound phospholipid; phosphatidylinositol 4,5 bisphosphate (PIP2) and disrupt its function. This distortion in PIP2 was independent of visual or non-visual G-protein coupled receptor activation. The change in PIP2 was followed by an increase in the cytosolic calcium, excessive cell shape change, and cell death. Blue light alone or retinal alone did not perturb PIP2 or elicit cytosolic calcium increase. Our data also suggest that photoexcited retinal-induced PIP2 distortion and subsequent oxidative damage incur in the core of the PM. These findings suggest that retinal exerts light sensitivity to both photoreceptor and non-photoreceptor cells, and intercepts crucial signaling events, altering the cellular fate.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

