Sir-2.1 mediated attenuation of α-synuclein expression by Alaskan bog blueberry polyphenols in a transgenic model of Caenorhabditis elegans
- PMID: 29976995
- PMCID: PMC6033853
- DOI: 10.1038/s41598-018-26905-4
Sir-2.1 mediated attenuation of α-synuclein expression by Alaskan bog blueberry polyphenols in a transgenic model of Caenorhabditis elegans
Abstract
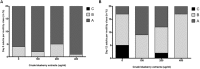
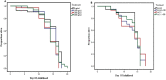
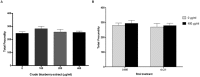
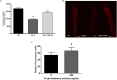
Misfolding and accumulation of cellular protein aggregates are pathological hallmarks of aging and neurodegeneration. One such protein is α-synuclein, which when misfolded, forms aggregates and disrupts normal cellular functions of the neurons causing Parkinson's disease. Nutritional interventions abundant in pharmacologically potent polyphenols have demonstrated a therapeutic role for combating protein aggregation associated with neurodegeneration. The current study hypothesized that Alaskan bog blueberry (Vaccinum uliginosum), which is high in polyphenolic content, will reduce α-synuclein expression in a model of Caenorhabditis elegans (C. elegans). We observed that blueberry extracts attenuated α-synuclein protein expression, improved healthspan in the form of motility and restored lipid content in the transgenic strain of C. elegans expressing human α-synuclein. We also found reduced gene expression levels of sir-2.1 (ortholog of mammalian Sirtuin 1) in blueberry treated transgenic animals indicating that the beneficial effects of blueberries could be mediated through partial reduction of sirtuin activity. This therapeutic effect of the blueberries was attributed to its xenohormetic properties. The current results highlight the role of Alaskan blueberries in mediating inhibition of sir-2.1 as a novel therapeutic approach to improving pathologies of protein misfolding diseases. Finally, our study warrants further investigation of the structure, and specificity of such small molecules from indigenous natural compounds and its role as sirtuin regulators.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

