Characterization and Validation of a Human 3D Cardiac Microtissue for the Assessment of Changes in Cardiac Pathology
- PMID: 29976997
- PMCID: PMC6033897
- DOI: 10.1038/s41598-018-28393-y
Characterization and Validation of a Human 3D Cardiac Microtissue for the Assessment of Changes in Cardiac Pathology
Abstract
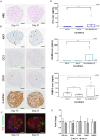
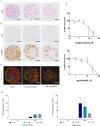
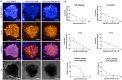
Pharmaceutical agents despite their efficacy to treat disease can cause additional unwanted cardiovascular side effects. Cardiotoxicity is characterized by changes in either the function and/or structure of the myocardium. Over recent years, functional cardiotoxicity has received much attention, however morphological damage to the myocardium and/or loss of viability still requires improved detection and mechanistic insights. A human 3D cardiac microtissue containing human induced pluripotent stem cell-derived cardiomyocytes (hiPS-CMs), cardiac endothelial cells and cardiac fibroblasts was used to assess their suitability to detect drug induced changes in cardiac structure. Histology and clinical pathology confirmed these cardiac microtissues were morphologically intact, lacked a necrotic/apoptotic core and contained all relevant cell constituents. High-throughput methods to assess mitochondrial membrane potential, endoplasmic reticulum integrity and cellular viability were developed and 15 FDA approved structural cardiotoxins and 14 FDA approved non-structural cardiotoxins were evaluated. We report that cardiac microtissues provide a high-throughput experimental model that is both able to detect changes in cardiac structure at clinically relevant concentrations and provide insights into the phenotypic mechanisms of this liability.
Conflict of interest statement
The authors declare no competing interests.
Figures
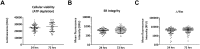


References
-
- Kawecka-Jaszcz K, Bednarek A. The management of cancer patients with heart disease. Przegl Lek. 2012;69:82–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

