The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects
- PMID: 29977074
- PMCID: PMC6098048
- DOI: 10.1038/s41386-018-0136-3
The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects
Abstract
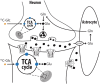
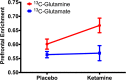
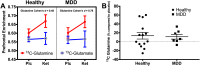
The ability of ketamine administration to activate prefrontal glutamate neurotransmission is thought to be a key mechanism contributing to its transient psychotomimetic effects and its delayed and sustained antidepressant effects. Rodent studies employing carbon-13 magnetic resonance spectroscopy (13C MRS) methods have shown ketamine and other N-methyl-D-aspartate (NMDA) receptor antagonists to transiently increase measures reflecting glutamate-glutamine cycling and glutamate neurotransmission in the frontal cortex. However, there are not yet direct measures of glutamate neurotransmission in vivo in humans to support these hypotheses. The current first-level pilot study employed a novel prefrontal 13C MRS approach similar to that used in the rodent studies for direct measurement of ketamine effects on glutamate-glutamine cycling. Twenty-one participants (14 healthy and 7 depressed) completed two 13C MRS scans during infusion of normal saline or subanesthetic doses of ketamine. Compared to placebo, ketamine increased prefrontal glutamate-glutamine cycling, as indicated by a 13% increase in 13C glutamine enrichment (t = 2.4, p = 0.02). We found no evidence of ketamine effects on oxidative energy production, as reflected by 13C glutamate enrichment. During ketamine infusion, the ratio of 13C glutamate/glutamine enrichments, a putative measure of neurotransmission strength, was correlated with the Clinician-Administered Dissociative States Scale (r = -0.54, p = 0.048). These findings provide the most direct evidence in humans to date that ketamine increases glutamate release in the prefrontal cortex, a mechanism previously linked to schizophrenia pathophysiology and implicated in the induction of rapid antidepressant effects.
Conflict of interest statement
Competing interests
CGA has served as a consultant and/or on advisory boards for Genentech and Janssen, and editor of “Chronic Stress” for Sage Publications, Inc. JHK is a consultant for AbbVie, Inc., Amgen, Astellas Pharma Global Development, Inc., AstraZeneca Pharmaceuticals, Biomedisyn Corporation, Bristol–Myers Squibb, Eli Lilly and Company, Euthymics Bioscience, Inc., Neurovance, Inc., FORUM Pharmaceuticals, Janssen Research & Development, Lundbeck Research USA, Novartis Pharma AG, Otsuka America Pharmaceutical, Inc., Sage Therapeutics, Inc., Sunovion Pharmaceuticals, Inc., and Takeda Industries. JHK is on the Scientific Advisory Board for Lohocla Research Corporation, Mnemosyne Pharmaceuticals, Inc., Naurex, Inc., and Pfizer. JHK is a stockholder in Biohaven Pharmaceuticals, holds stock options in Mnemosyne Pharmaceuticals, Inc., holds patents for Dopamine and Noradrenergic Reuptake Inhibitors in Treatment of Schizophrenia, U.S. Patent No. 5,447,948 (issued September 5, 1995), and Glutamate Modulating Agents in the Treatment of Mental Disorders, U.S. Patent No. 8,778,979 (issued July 15, 2014); and filed a patent for Intranasal Administration of Ketamine to Treat Depression. U.S. Application No. 14/197,767 (filed on March 5, 2014); U.S. application or Patent Cooperation Treaty international application No. 14/306,382 (filed on June 17, 2014). GS reports personal consulting fees from Alkermes, Allergan, Biohaven Pharmaceuticals, Eli Lilly and Co., Genentech, Intra-Cellular Therapies, Janssen Pharmaceuticals, Lundbeck Research USA, Merck & Co., Naurex, Navitor Pharmaceuticals, Noven Pharmaceuticals, Teva Pharmaceuticals Industries, Taisho Pharmaceutical Co., Takeda Pharmaceutical Co., Sage Pharmaceuticals, Inc., Sevier, Valeant Pharmaceuticals, and Vistagen Therapeutics, Inc. GS has grants and research contracts from Eli Lilly and Co., Janssen Pharmaceuticals, Merck & Co., and Sevier; and support from Sanofi-Aventis, in the form of free medication for an NIH sponsored study over the last 36 months. In addition, Dr. GS is a stockholder and holds stock options in Biohaven Pharmaceuticals; and has a patent for Glutamate Modulating Agents in the Treatment of Mental Disorders, U.S. Patent No. 8,778,979 (issued July 15, 2014) with royalties paid from Biohaven Pharmaceuticals. GFM is a consultant for Sumitomo Dainippon Pharma Co. Ltd. and UCB Pharma SA, and serves on the Scientific Advisory Board of Elucidata, Inc. All other authors report no competing interests.
Ethics approval
All study procedures were approved by an institutional review board (ClinicalTrials.gov NCT02037035).
Figures


References
-
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

