Nonallergic Asthma and Its Severity: Biomarkers for Its Discrimination in Peripheral Samples
- PMID: 29977241
- PMCID: PMC6021512
- DOI: 10.3389/fimmu.2018.01416
Nonallergic Asthma and Its Severity: Biomarkers for Its Discrimination in Peripheral Samples
Abstract
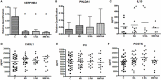
Asthma is a complex and heterogeneous respiratory disorder characterized by chronic airway inflammation. It has generally been associated with allergic mechanisms related to type 2 airway inflammation. Nevertheless, between 10 and 33% of asthmatic individuals have nonallergic asthma (NA). Several targeted treatments are in clinical development for patients with Th2 immune response, but few biomarkers are been defined for low or non-Th2-mediated inflammation asthma. We have recently defined by gene expression a set of genes as potential biomarkers of NA, mainly associated with disease severity: IL10, MSR1, PHLDA1, SERPINB2, CHI3L1, IL8, and PI3. Here, we analyzed their protein expression and specificity using sera and isolated peripheral blood mononuclear cells (PBMCs). First, protein quantification was carried out using ELISA (in sera) or Western blot (proteins extracted from PBMCs by Trizol procedure), depending on the biomarker in 30 healthy controls (C) subjects and 30 NA patients. A receiver operating characteristic curve analysis was performed by using the R program to study the specificity and sensitivity of the candidate biomarkers at a gene- and protein expression level. Four kinds of comparisons were performed: total NA group vs C group, severe NA patients vs C, moderate-mild NA patients vs C, and severe NA patients vs moderate-mild NA patients. We found that all the single genes showed good sensitivity vs specificity for some phenotypic discrimination, with CHI3L1 and PI3 exhibiting the best results for C vs NA: CHI3L1 area under the curve (AUC) (CI 95%): 0.95 (0.84-1.00) and PI3 AUC: 0.99 (0.98-1.00); C vs severe NA: PI3 AUC: 1 (0.99-1.00); and C vs moderate-mild NA: CHI3L1 AUC: 1 (0.99-1.00) and PI3 AUC: 0.99 (0.96-1.00). However, the results for discriminating asthma disease and severity with protein expression were better when two or three biomarkers were combined. In conclusion, individual genes and combinations of proteins have been evaluated as reliable biomarkers for classifying NA subjects and their severity. These new panels could be good diagnostic tests.
Keywords: biomarkers; gene expression; nonallergic asthma; protein expression; receiver operating characteristic; severity.
Figures
References
-
- GINA. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. (2017). Available from: http://www.ginasthma.org/
LinkOut - more resources
Full Text Sources
Other Literature Sources

