Heterozygous CDKL5 Knockout Female Mice Are a Valuable Animal Model for CDKL5 Disorder
- PMID: 29977282
- PMCID: PMC5994305
- DOI: 10.1155/2018/9726950
Heterozygous CDKL5 Knockout Female Mice Are a Valuable Animal Model for CDKL5 Disorder
Abstract
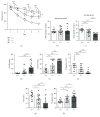
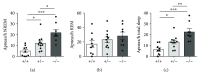
CDKL5 disorder is a severe neurodevelopmental disorder caused by mutations in the X-linked CDKL5 (cyclin-dependent kinase-like five) gene. CDKL5 disorder primarily affects girls and is characterized by early-onset epileptic seizures, gross motor impairment, intellectual disability, and autistic features. Although all CDKL5 female patients are heterozygous, the most valid disease-related model, the heterozygous female Cdkl5 knockout (Cdkl5 +/-) mouse, has been little characterized. The lack of detailed behavioral profiling of this model remains a crucial gap that must be addressed in order to advance preclinical studies. Here, we provide a behavioral and molecular characterization of heterozygous Cdkl5 +/- mice. We found that Cdkl5 +/- mice reliably recapitulate several aspects of CDKL5 disorder, including autistic-like behaviors, defects in motor coordination and memory performance, and breathing abnormalities. These defects are associated with neuroanatomical alterations, such as reduced dendritic arborization and spine density of hippocampal neurons. Interestingly, Cdkl5 +/- mice show age-related alterations in protein kinase B (AKT) and extracellular signal-regulated kinase (ERK) signaling, two crucial signaling pathways involved in many neurodevelopmental processes. In conclusion, our study provides a comprehensive overview of neurobehavioral phenotypes of heterozygous female Cdkl5 +/- mice and demonstrates that the heterozygous female might be a valuable animal model in preclinical studies on CDKL5 disorder.
Figures






Similar articles
-
Autistic-relevant behavioral phenotypes of a mouse model of cyclin-dependent kinase-like 5 deficiency disorder.Autism Res. 2024 Sep;17(9):1742-1759. doi: 10.1002/aur.3226. Epub 2024 Sep 5. Autism Res. 2024. PMID: 39234879
-
X-linked cellular mosaicism underlies age-dependent occurrence of seizure-like events in mouse models of CDKL5 deficiency disorder.Neurobiol Dis. 2021 Jan;148:105176. doi: 10.1016/j.nbd.2020.105176. Epub 2020 Nov 13. Neurobiol Dis. 2021. PMID: 33197557 Free PMC article.
-
CDKL5 protein substitution therapy rescues neurological phenotypes of a mouse model of CDKL5 disorder.Hum Mol Genet. 2018 May 1;27(9):1572-1592. doi: 10.1093/hmg/ddy064. Hum Mol Genet. 2018. PMID: 29474534
-
Molecular and Synaptic Bases of CDKL5 Disorder.Dev Neurobiol. 2019 Jan;79(1):8-19. doi: 10.1002/dneu.22639. Epub 2018 Oct 19. Dev Neurobiol. 2019. PMID: 30246934 Review.
-
Cyclin-Dependent Kinase-Like 5 (CDKL5): Possible Cellular Signalling Targets and Involvement in CDKL5 Deficiency Disorder.Neural Plast. 2020 Jun 5;2020:6970190. doi: 10.1155/2020/6970190. eCollection 2020. Neural Plast. 2020. PMID: 32587608 Free PMC article. Review.
Cited by
-
Aged heterozygous Cdkl5 mutant mice exhibit spontaneous epileptic spasms.Exp Neurol. 2020 Oct;332:113388. doi: 10.1016/j.expneurol.2020.113388. Epub 2020 Jun 22. Exp Neurol. 2020. PMID: 32585155 Free PMC article.
-
Therapeutic potential of pregnenolone and pregnenolone methyl ether on depressive and CDKL5 deficiency disorders: Focus on microtubule targeting.J Neuroendocrinol. 2022 Feb;34(2):e13033. doi: 10.1111/jne.13033. Epub 2021 Sep 8. J Neuroendocrinol. 2022. PMID: 34495563 Free PMC article. Review.
-
Beneficial Antioxidant Effects of Coenzyme Q10 in In Vitro and In Vivo Models of CDKL5 Deficiency Disorder.Int J Mol Sci. 2025 Feb 28;26(5):2204. doi: 10.3390/ijms26052204. Int J Mol Sci. 2025. PMID: 40076840 Free PMC article.
-
Transcriptomic Profiling of Zebrafish Mutant for cdkl5 Reveals Dysregulated Gene Expression Associated with Neuronal, Muscle, Visual and Skeletal Development.Int J Mol Sci. 2025 Jun 24;26(13):6069. doi: 10.3390/ijms26136069. Int J Mol Sci. 2025. PMID: 40649845 Free PMC article.
-
Site-specific abnormalities in the visual system of a mouse model of CDKL5 deficiency disorder.Hum Mol Genet. 2019 Sep 1;28(17):2851-2861. doi: 10.1093/hmg/ddz102. Hum Mol Genet. 2019. PMID: 31108505 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

