Evaluation of Oxidative Stress and Mitophagy during Adipogenic Differentiation of Adipose-Derived Stem Cells Isolated from Equine Metabolic Syndrome (EMS) Horses
- PMID: 29977307
- PMCID: PMC6011082
- DOI: 10.1155/2018/5340756
Evaluation of Oxidative Stress and Mitophagy during Adipogenic Differentiation of Adipose-Derived Stem Cells Isolated from Equine Metabolic Syndrome (EMS) Horses
Abstract
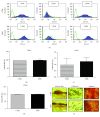
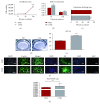
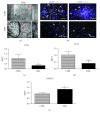
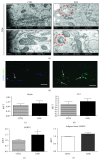
Mesenchymal stem cells (MSCs) are frequently used in both human and veterinary medicine because their unique properties, such as modulating the immune response and differentiating into multiple lineages, make them a valuable tool in cell-based therapies. However, many studies have indicated the age-, lifestyle-, and disease-related deterioration of MSC regenerative characteristics. However, it still needs to be elucidated how the patient's health status affects the effectiveness of MSC differentiation. In the present study, we isolated mesenchymal stem cells from adipose tissue (adipose-derived mesenchymal stem cells (ASCs)) from horses diagnosed with equine metabolic syndrome (EMS), a common metabolic disorder characterized by pathological obesity and insulin resistance. We investigated the metabolic status of isolated cells during adipogenic differentiation using multiple research methods, such as flow cytometry, PCR, immunofluorescence, or transmission and confocal microscopy. The results indicated the impaired differentiation potential of ASCEMS. Excessive ROS accumulation and ER stress are most likely the major factors limiting the multipotency of these cells. However, we observed autophagic flux during differentiation as a protective mechanism that allows cells to maintain homeostasis and remove dysfunctional mitochondria.
Figures



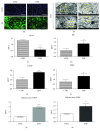
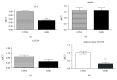
References
-
- Marędziak M., Marycz K., Lewandowski D., Siudzińska A., Śmieszek A. Static magnetic field enhances synthesis and secretion of membrane-derived microvesicles (MVs) rich in VEGF and BMP-2 in equine adipose-derived stromal cells (EqASCs)—a new approach in veterinary regenerative medicine. In Vitro Cellular & Developmental Biology - Animal. 2015;51(3):230–240. doi: 10.1007/s11626-014-9828-0. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

