Acyl-group specificity of AHL synthases involved in quorum-sensing in Roseobacter group bacteria
- PMID: 29977398
- PMCID: PMC6009203
- DOI: 10.3762/bjoc.14.112
Acyl-group specificity of AHL synthases involved in quorum-sensing in Roseobacter group bacteria
Abstract
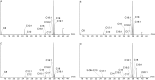
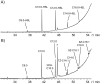
N-Acylhomoserine lactones (AHLs) are important bacterial messengers, mediating different bacterial traits by quorum sensing in a cell-density dependent manner. AHLs are also produced by many bacteria of the marine Roseobacter group, which constitutes a large group within the marine microbiome. Often, specific mixtures of AHLs differing in chain length and oxidation status are produced by bacteria, but how the biosynthetic enzymes, LuxI homologs, are selecting the correct acyl precursors is largely unknown. We have analyzed the AHL production in Dinoroseobacter shibae and three Phaeobacter inhibens strains, revealing strain-specific mixtures. Although large differences were present between the species, the fatty acid profiles, the pool for the acyl precursors for AHL biosynthesis, were very similar. To test the acyl-chain selectivity, the three enzymes LuxI1 and LuxI2 from D. shibae DFL-12 as well as PgaI2 from P. inhibens DSM 17395 were heterologously expressed in E. coli and the enzymes isolated for in vitro incubation experiments. The enzymes readily accepted shortened acyl coenzyme A analogs, N-pantothenoylcysteamine thioesters of fatty acids (PCEs). Fifteen PCEs were synthesized, varying in chain length from C4 to C20, the degree of unsaturation and also including unusual acid esters, e.g., 2E,11Z-C18:2-PCE. The latter served as a precursor of the major AHL of D. shibae DFL-12 LuxI1, 2E,11Z-C18:2-homoserine lactone (HSL). Incubation experiments revealed that PgaI2 accepts all substrates except C4 and C20-PCE. Competition experiments demonstrated a preference of this enzyme for C10 and C12 PCEs. In contrast, the LuxI enzymes of D. shibae are more selective. While 2E,11Z-C18:2-PCE is preferentially accepted by LuxI1, all other PCEs were not, except for the shorter, saturated C10-C14-PCEs. The AHL synthase LuxI2 accepted only C14 PCE and 3-hydroxydecanoyl-PCE. In summary, chain-length selectivity in AHLs can vary between different AHL enzymes. Both, a broad substrate acceptance and tuned specificity occur in the investigated enzymes.
Keywords: Dinoroseobacter shibae; N-acylhomoserine lactones; Phaeobacter inhibens; fatty acid composition; quorum sensing.
Figures
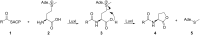

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
