Synthesis of chiral 3-substituted 3-amino-2-oxindoles through enantioselective catalytic nucleophilic additions to isatin imines
- PMID: 29977400
- PMCID: PMC6009130
- DOI: 10.3762/bjoc.14.114
Synthesis of chiral 3-substituted 3-amino-2-oxindoles through enantioselective catalytic nucleophilic additions to isatin imines
Abstract
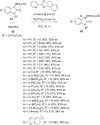
This review collects the recent developments in the synthesis of chiral 3-substituted 3-amino-2-oxindoles based on enantioselective catalytic nucleophilic additions to isatin imines published since the beginning of 2015.
Keywords: asymmetric synthesis; chiral 3-amino-2-oxindoles; chirality; isatin imines; nucleophilic addition.
Figures

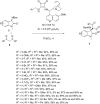
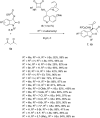
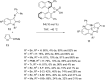

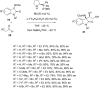





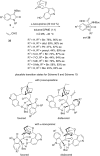




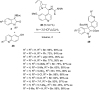

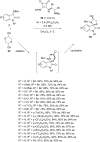
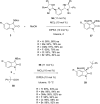
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
