The relationship between the expression of thymidylate synthase, dihydropyrimidine dehydrogenase, orotate phosphoribosyltransferase, excision repair cross-complementation group 1 and class III β-tubulin, and the therapeutic effect of S-1 or carboplatin plus paclitaxel in non-small-cell lung cancer
- PMID: 29977535
- PMCID: PMC6031014
- DOI: 10.3892/mco.2018.1619
The relationship between the expression of thymidylate synthase, dihydropyrimidine dehydrogenase, orotate phosphoribosyltransferase, excision repair cross-complementation group 1 and class III β-tubulin, and the therapeutic effect of S-1 or carboplatin plus paclitaxel in non-small-cell lung cancer
Abstract
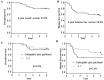
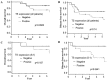
Previous studies have reported that the expressions of specific proteins may predict the efficacy of chemotherapy agents for non-small cell lung cancer (NSCLC) patients. The present study evaluated the expression of proteins hypothesized to be associated with the effect of chemotherapeutic agents in 38 NSCLC patients with pathological stage II and IIIA. The subjects received carboplatin plus paclitaxel (CP) or S-1 as adjuvant chemotherapy following complete resection. The protein expressions evaluated were those of thymidylate synthase (TS), dihydropyrimidine dehydrogenase (DPD) and orotate phsphoribosyltransferase (OPRT), which were suspected to be associated with the effect of S-1 agents, excision repair cross-complementation group 1 (ERCC1), which was suspected to be associated with the effect of platinum-based agents, and class III β-tubulin (TUBB3), which was suspected to be associated with the effect of taxane-based agents. The positive rate of TS was 55.3% (n=21/38), DPD was 57.9% (n=22/38), OPRT was 42.1% (n=16/38), ERCC1 was 47.4% (n=18/38) and TUBB3 was 44.7% (n=17/38). Among the patients who received S-1 adjuvant chemotherapy, TS-negative cases demonstrated a significantly better disease-free survival than positive cases. Thus, TS protein expression may have been a factor that predicted the effect of S-1 agent as adjuvant chemotherapy.
Keywords: adjuvant chemotherapy; class III β-tubulin; dihydropyrimidine dehydrogenase; excision repair cross-complementation group 1; non-small cell lung cancer; orotate phsphoribosyltransferase; protein expression; thymidylate synthase.
Figures

References
-
- Detterbeck FC, Chansky K, Groome P, Bolejack V, Crowley J, Shemnski L, Kennedy C, Krasnik M, Peake M, Rami-Porta R, et al. The IASLC lung cancer staging project: Methodology and validation used in the development of proposals for revision of the stage classification of NSCLC in the forthcoming (eighth) edition of the TNM classification of lung cancer. J Thorac Oncol. 2016;11:1433–1446. doi: 10.1016/j.jtho.2016.01.024. - DOI - PubMed
-
- Kis MG, Gaspar LE, Chaft JE, Kennedy EB, Azzoli CG, Ellis PM, Lin SH, Pass HI, Seth R, Shepherd FA, et al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American society of clinical oncology/cancer care ontario clinical practice guideline update. J Clin Oncol. 2017;35:2960–2974. doi: 10.1200/JCO.2017.72.4401. - DOI - PubMed
-
- Pisters KM, Evans WK, Azzoli CG, Kris MG, Smith CA, Desch CE, Somerfield MR, Brouwers MC, Darling G, Ellis PM, et al. Cancer care onario and American society of clinical oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I–IIIA resectable non-small cell lung cancer guideline. J Clin Oncol. 2007;25:5506–5518. doi: 10.1200/JCO.2007.14.1226. - DOI - PubMed
-
- Crinò L, Weder W, van Meerbeeck J, Felip E. ESMO Guidelines Working Group: Early stage and locally acvanced (non-metastatic) non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v103–v115. doi: 10.1093/annonc/mdq207. - DOI - PubMed
-
- NSCLC Meta-analyses Collaborative Group, corp-author. Arriagada R, Auperin A, Burdett S, Higgins JP, Johnson DH, Le Chevalier T, Le Pechoux C, Parmar MK, Pignon JP, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: Two meta-analyses of individual patient data. Lancet. 2010;375:1267–1277. doi: 10.1016/S0140-6736(10)60059-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
