Integration of Transcriptomic and Proteomic Approaches Reveals the Temperature-Dependent Virulence of Pseudomonas plecoglossicida
- PMID: 29977868
- PMCID: PMC6021524
- DOI: 10.3389/fcimb.2018.00207
Integration of Transcriptomic and Proteomic Approaches Reveals the Temperature-Dependent Virulence of Pseudomonas plecoglossicida
Abstract
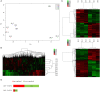
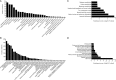
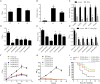
Pseudomonas plecoglossicida is a facultative pathogen that is associated with diseases of multiple fish, mainly at 15-20°C. Although fish disease caused by P. plecoglossicida has led to significant economic losses, the mechanisms of the temperature-dependent virulence are unclear. Here, we identify potential pathogenicity mechanisms and demonstrate the direct regulation of several virulence factors by temperature with transcriptomic and proteomic analyses, quantitative real-time PCR (qRT-PCR), RNAi, pyoverdine (PVD) quantification, the chrome azurol S (CAS) assay, growth curve measurements, a biofilm assay, and artificial infection. The principal component analysis, the heat map generation and hierarchical clustering, together with the functional annotations of the differentially expressed genes (DEGs) demonstrated that, under different growth temperatures, the animation and focus of P. plecoglossicida are quite different, which may be the key to pathogenicity. Genes involved in PVD synthesis and in the type VI secretion system (T6SS) are specifically upregulated at the virulent temperature of 18°C. Silencing of the PVD-synthesis-related genes reduces the iron acquisition, growth, biofilm formation, distribution in host organs and virulence of the bacteria. Silencing of the T6SS genes also leads to the reduction of biofilm formation, distribution in host organs and virulence. These findings reveal that temperature regulates multiple virulence mechanisms in P. plecoglossicida, especially through iron acquisition and T6SS secretion. Meanwhile, integration of transcriptomic and proteomic data provide us with a new perspective into the pathogenesis of P. plecoglossicida, which would not have been easy to catch at either the protein or mRNA differential analyses alone, thus illustrating the power of multi-omics analyses in microbiology.
Keywords: Pseudomonas plecoglossicida; proteomics; temperature; transcriptome; virulence; white nodules disease.
Figures



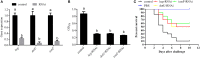
Similar articles
-
Temperature-regulated expression of type VI secretion systems in fish pathogen Pseudomonas plecoglossicida revealed by comparative secretome analysis.FEMS Microbiol Lett. 2016 Nov;363(22):fnw261. doi: 10.1093/femsle/fnw261. Epub 2016 Nov 13. FEMS Microbiol Lett. 2016. PMID: 27974393
-
Integration of RNAi and RNA-seq uncovers the immune responses of Epinephelus coioides to L321_RS19110 gene of Pseudomonas plecoglossicida.Fish Shellfish Immunol. 2018 Oct;81:121-129. doi: 10.1016/j.fsi.2018.06.051. Epub 2018 Jul 10. Fish Shellfish Immunol. 2018. PMID: 30006040
-
Dual RNA-Seq Unveils Pseudomonas plecoglossicida htpG Gene Functions During Host-Pathogen Interactions With Epinephelus coioides.Front Immunol. 2019 May 3;10:984. doi: 10.3389/fimmu.2019.00984. eCollection 2019. Front Immunol. 2019. PMID: 31130962 Free PMC article.
-
The bacterial pathogen Pseudomonas plecoglossicida, its epidemiology, virulence factors, vaccine development, and host-pathogen interactions.J Aquat Anim Health. 2024 Jun;36(2):181-191. doi: 10.1002/aah.10215. Epub 2024 Feb 25. J Aquat Anim Health. 2024. PMID: 38402543 Review.
-
The T6SSs of Pseudomonas aeruginosa Strain PAO1 and Their Effectors: Beyond Bacterial-Cell Targeting.Front Cell Infect Microbiol. 2016 Jun 9;6:61. doi: 10.3389/fcimb.2016.00061. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27376031 Free PMC article. Review.
Cited by
-
Anti-Oomycete Activity and Pepper Root Colonization of Pseudomonas plecoglossicida YJR13 and Pseudomonas putida YJR92 against Phytophthora capsici.Plant Pathol J. 2023 Feb;39(1):123-135. doi: 10.5423/PPJ.OA.01.2023.0001. Epub 2023 Feb 1. Plant Pathol J. 2023. PMID: 36760054 Free PMC article.
-
Dual RNA-seq provides novel insight into the roles of dksA from Pseudomonas plecoglossicida in pathogen-host interactions with large yellow croakers ( Larimichthys crocea).Zool Res. 2020 Jul 18;41(4):410-422. doi: 10.24272/j.issn.2095-8137.2020.048. Zool Res. 2020. PMID: 32521576 Free PMC article.
-
Molecular characterization, expression pattern and immunologic function of CD82a in large yellow croaker (Larimichthys crocea).Front Immunol. 2024 Feb 2;15:1301877. doi: 10.3389/fimmu.2024.1301877. eCollection 2024. Front Immunol. 2024. PMID: 38370405 Free PMC article.
-
The regulation of oxidative phosphorylation pathway on Vibrio alginolyticus adhesion under adversities.Microbiologyopen. 2019 Aug;8(8):e00805. doi: 10.1002/mbo3.805. Epub 2019 Feb 14. Microbiologyopen. 2019. PMID: 30767412 Free PMC article.
-
Mechanisms Underlying the Virulence Regulation of Vibrio alginolyticus ND-01 pstS and pstB with a Transcriptomic Analysis.Microorganisms. 2022 Oct 22;10(11):2093. doi: 10.3390/microorganisms10112093. Microorganisms. 2022. PMID: 36363689 Free PMC article.
References
-
- Aguilera S., Alvarez-Morales A., Murillo J., Hernández-Flores J. L., Bravo J., De la Torre-Zavala S. (2017). Temperature-mediated biosynthesis of the phytotoxin phaseolotoxin by Pseudomonas syringae pv. phaseolicola depends on the autoregulated expression of the phtABC genes. PLoS ONE 12:e0178441. 10.1371/journal.pone.0178441 - DOI - PMC - PubMed
-
- Akayli T., Canak O., Basaran B. (2011). A new Pseudomonas species observed in cultured young rainbow trout (Oncorhynchus mykiss Walbaum, 1792): Pseudomonas plecoglossicida. Res. J. Biol. Sci. 4, 107–111.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

