One-Step Synthesis of Hexagonal Boron Nitrides, Their Crystallinity and Biodegradation
- PMID: 29977891
- PMCID: PMC6021499
- DOI: 10.3389/fbioe.2018.00083
One-Step Synthesis of Hexagonal Boron Nitrides, Their Crystallinity and Biodegradation
Abstract
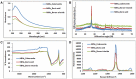
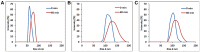
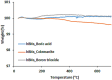
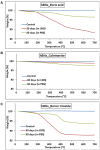
Hexagonal boron nitrides (hBNs) have recently been investigated for several novel applications due to their unique properties such as biocompatibility, superhydrophobicity, electrical insulation, and thermal and chemical stability. In addition, their biodegradation products have recently reported to have therapeutic effect on certain cancer types. hBNs are easily synthesized from boron and nitrogen precursors at moderately low temperatures. However, crystallinity and yield vary depending on the type of precursor, reaction temperature, and duration. In this study, a simple one-step hBNs synthesis method is reported without a catalyst, which might be an undesired contaminant for biomedical applications. The influence of boron precursors (boric acid, colemanite, or boron trioxide) on hBNs crystallinity, stability, and biodegradation in suspensions containing oxidative and hydrolytic degradation agents is investigated with the aim of their possible application in biomedicine. We found that the choice of boron precursor is a critically important parameter controlling the hBNs crystallinity and dependently influencing the biodegradation rate.
Keywords: biodegradation; boric acid; boron trioxide; colemanite; hexagonal boron nitride; synthesis.
Figures



Similar articles
-
Synthesis, Functionalization, and Bioapplications of Two-Dimensional Boron Nitride Nanomaterials.Front Bioeng Biotechnol. 2019 Dec 10;7:363. doi: 10.3389/fbioe.2019.00363. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31921797 Free PMC article. Review.
-
Stimulatory Effect of Hexagonal Boron Nitrides in Wound Healing.ACS Appl Bio Mater. 2019 Dec 16;2(12):5582-5596. doi: 10.1021/acsabm.9b00669. Epub 2019 Nov 18. ACS Appl Bio Mater. 2019. PMID: 35021553
-
Hexagonal boron nitrides reduce the oxidative stress on cells.Nanotechnology. 2020 May 22;31(21):215101. doi: 10.1088/1361-6528/ab6fdc. Epub 2020 Jan 24. Nanotechnology. 2020. PMID: 31978926
-
Evaluation of boron nitride nanotubes and hexagonal boron nitrides as nanocarriers for cancer drugs.Nanomedicine (Lond). 2017 Apr;12(7):797-810. doi: 10.2217/nnm-2016-0322. Epub 2017 Mar 21. Nanomedicine (Lond). 2017. PMID: 28322118
-
Boron nitride nanotubes: synthesis and applications.Nano Converg. 2018;5(1):17. doi: 10.1186/s40580-018-0149-y. Epub 2018 Jun 28. Nano Converg. 2018. PMID: 30046512 Free PMC article. Review.
Cited by
-
Synthesis, Functionalization, and Bioapplications of Two-Dimensional Boron Nitride Nanomaterials.Front Bioeng Biotechnol. 2019 Dec 10;7:363. doi: 10.3389/fbioe.2019.00363. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31921797 Free PMC article. Review.
-
Microglia Polarization and Antiglioma Effects Fostered by Dual Cell Membrane-Coated Doxorubicin-Loaded Hexagonal Boron Nitride Nanoflakes.ACS Appl Mater Interfaces. 2023 Dec 20;15(50):58260-58273. doi: 10.1021/acsami.3c17097. Epub 2023 Dec 5. ACS Appl Mater Interfaces. 2023. PMID: 38051559 Free PMC article.
-
pH-Controlled fluorescence switching in water-dispersed polymer brushes grafted to modified boron nitride nanotubes for cellular imaging.Beilstein J Nanotechnol. 2019 Dec 10;10:2428-2439. doi: 10.3762/bjnano.10.233. eCollection 2019. Beilstein J Nanotechnol. 2019. PMID: 31921521 Free PMC article.
-
Ameliorative Effects by Hexagonal Boron Nitride Nanoparticles against Beta Amyloid Induced Neurotoxicity.Nanomaterials (Basel). 2022 Aug 5;12(15):2690. doi: 10.3390/nano12152690. Nanomaterials (Basel). 2022. PMID: 35957121 Free PMC article.
-
Synergistic anti-cancer effects of piezoelectric hexagonal boron nitride nanocarriers for controlled doxorubicin release.Nanomedicine (Lond). 2025 Mar;20(5):455-466. doi: 10.1080/17435889.2025.2459055. Epub 2025 Jan 31. Nanomedicine (Lond). 2025. PMID: 39887263
References
-
- Arenal R., Lopez-Bezanilla A. (2015). Boron nitride materials: an overview from 0D to 3D (nano) structures. Wiley Interdisciplin. Rev. Comput. Mol. Sci. 5, 299–309. 10.1002/wcms.1219 - DOI
-
- Bayca S. U., Kocan F., Abali Y. (2014). Dissolution of colemanite process waste in oxalic acid solutions. Environ. Prog. Sustain. Energy 33, 1111–1116. 10.1002/ep.11889 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

