Peak MSC-Are We There Yet?
- PMID: 29977893
- PMCID: PMC6021509
- DOI: 10.3389/fmed.2018.00178
Peak MSC-Are We There Yet?
Abstract
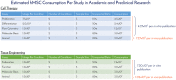
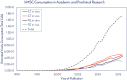
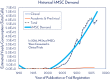
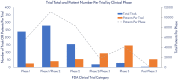
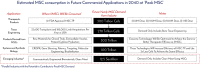
Human mesenchymal stem cells (hMSCs) are a critical raw material for many regenerative medicine products, including cell-based therapies, engineered tissues, or combination products, and are on the brink of radically changing how the world of medicine operates. Their unique characteristics, potential to treat many indications, and established safety profile in more than 800 clinical trials have contributed to their current consumption and will only fuel future demand. Given the large target patient populations with typical dose sizes of 10's to 100's of millions of cells per patient, and engineered tissues being constructed with 100's of millions to billions of cells, an unprecedented demand has been created for hMSCs. The fulfillment of this demand faces an uphill challenge in the limited availability of large quantities of pharmaceutical grade hMSCs for the industry-fueling the need for parallel rapid advancements in the biomanufacturing of this living critical raw material. Simply put, hMSCs are no different than technologies like transistors, as they are a highly technical and modular product that requires stringent control over manufacturing that can allow for high quality and consistent performance. As hMSC manufacturing processes are optimized, it predicts a future time of abundance for hMSCs, where scientists and researchers around the world will have access to a consistent and readily available supply of high quality, standardized, and economical pharmaceutical grade product to buy off the shelf for their applications and drive product development-this is "Peak MSC."
Keywords: bioprocessing; cell manufacturing; cell therapy; mesenchymal stem cell; regenerative medicine; stem cell.
Figures





References
-
- Maris B. Medicine's transistor moment 8 emerging technologies that could revolutionize the life sciences. Medium (2015) Available online at: https://library.gv.com/medicine-s-transistor-moment-fb6c88f4352f
-
- Diamandis PH, Kotler S, Diamandis PH, Abundance: The Future Is Better Than You Think. (2012). Available online at: http://www.amazon.com/abundance-future-better-than-think/dp/1451614217/r...
-
- Carlson RH. Biology is technology: the promise, peril, and new business of engineering life. J Evol Technol. (2010) 304:288. 10.1001/jama.2010.1552 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

