Summary of the international clinical guidelines for the management of hospital-acquired and ventilator-acquired pneumonia
- PMID: 29977898
- PMCID: PMC6018155
- DOI: 10.1183/23120541.00028-2018
Summary of the international clinical guidelines for the management of hospital-acquired and ventilator-acquired pneumonia
Abstract
A summary of the evidence and recommendations made in the ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia http://ow.ly/S3zA30iZfLa.
Conflict of interest statement
Conflict of interest: A. Torres reports grants from MedImmune, Cubist, Bayer, Theravance, and from Poliphor; personal fees from Bayer (Advisory Board), Roche (Advisory Board), The Medicines CO (Advisory Board), and from Curetis (rapid molecular tests) (Advisory Board); grants as the Principal Investigator of a Phase III study of Cefatzidime/Avibactam for HAP/VAP, during the conduct of the study. He has received personal fees from GSK (Educational symposium: Siglo XXI), Pfizer (speaker at sponsored vaccine symposia), Astra Zeneca (speaker at symposia on ceftaroline), and from Biotest Advisory Board (Enriched IgM for severe CAP), outside the submitted work. Conflict of interest: M.S. Niederman reports grants and personal fees from Bayer (inhaled amikacin research grant and consulting) and Merck (nosocomial pneumonia consulting and grant), personal fees from Thermo-Fisher (procalcitonin lectures), Theravance (consulting on telavancin), and Pfizer (consulting on linezolid), during the conduct of the study. Conflict of interest: J. Chastre reports personal fees from Medimmune/AstraZeneca (honoraria for consulting), Bayer (honoraria for consulting), Pfizer (honoraria for lecture), Cubist/Merck (honoraria for data safety committee participation), Aridis (honoraria for consulting and data safety participation), and Astellas (honoraria for consulting), outside the submitted work. Conflict of interest: G. Li Bassi reports grants from Medimmune, Cubist, Covidien, Theravance, Cardeas Pharma, Ciel Medical, Biovo Technologies, and Toray, during the conduct of the study. Conflict of interest: C. Luna reports grants and personal fees from Pfizer, and personal fees from Merck Sharp and Dohne, outside the submitted work. Conflict of interest: D. Rigau is a European Respiratory Society methodologist. Conflict of interest: T. Welte reports personal fees (for lectures/advisory board) from AstraZeneca, Basilea, Bayer, Grifols, Infectopharm, Novartis and Pfizer, research grants from Bayer, Grifols, Novartis and Pfizer, research grants from the EU, German Research Council and German Ministry of Research and Education, during the conduct of the study. He has received personal fees (for lectures/advisory board) from Boehringer, Berlin Chemie, Mundipharma and Insmed, outside the submitted work. Conflict of interest: R. Wunderink reports personal fees from Arsanis, Accelerate Diagnostics, Bayer, Cepheid, GenMark, The Medicines Company, Merck, Nabriva, Polyphor and Shionogi, during the conduct of the study. Conflict of interest: M. Kollef reports personal fees from Accelerate (consultant), Merck (consultant, speaker's bureau), Paratek (consultant) and Allergan (speaker's bureau), outside the submitted work. Conflict of interest: J.F. Timsit reports research grants from Merck and 3M, a research grant to his hospital from Astelas, and educational grants from Gilead; and personal fees (for advisory boards) from Pfizer, Paratek, Merck, Bayer, 3M and Abbott, all outside the submitted work.
Figures
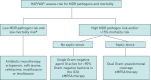
Comment in
-
Treating nosocomial pneumonia: what's new.ERJ Open Res. 2018 Jun 26;4(2):00058-2018. doi: 10.1183/23120541.00058-2018. eCollection 2018 Apr. ERJ Open Res. 2018. PMID: 29978856 Free PMC article.
References
-
- Salluh JIF, Souza-Dantas VC, Póvoa P. The current status of biomarkers for the diagnosis of nosocomial pneumonias. Curr Opin Crit Care 2017; 23: 391–397. - PubMed
-
- Torres A, Niederman MS, Chastre J, et al. . International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur Respir J 2017; 50: 1700582. - PubMed
-
- Blanquer J, Aspa J, Anzueto A, et al. . Normativa SEPAR: neumonía nosocomial [SEPAR guidelines for nosocomial pneumonia]. Arch Bronconeumol 2011; 47: 510–520. - PubMed
-
- Díaz E, Martín-Loeches I, Vallés J. Neumonía nosocomial [Nosocomial pneumonia]. Enferm Infecc Microbiol Clin 2013; 31: 692–698. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
