FNDC4 Inhibits RANKL-Induced Osteoclast Formation by Suppressing NF- κ B Activation and CXCL10 Expression
- PMID: 29977911
- PMCID: PMC5998196
- DOI: 10.1155/2018/3936257
FNDC4 Inhibits RANKL-Induced Osteoclast Formation by Suppressing NF- κ B Activation and CXCL10 Expression
Abstract
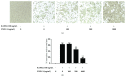
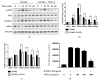
FNDC4 acts as an anti-inflammatory factor on macrophages and improves mouse model of induced colitis. Considering osteoclast formation is characterized by the activation of inflammation-related pathways, we thus speculated that FNDC4 may play a pivotal role in this process. RT-qPCR analysis was performed to confirm the expression of osteoclast formation related genes in primary murine bone marrow macrophages (BMMs). RANKL-treated BMMs were cultured with FNDC4 to evaluate the effect of FNDC4 on osteoclast differentiation. TRAP staining and bone resorption pits assay were used to assess osteoclast formation and bone resorption, respectively. Luciferase assay and western blotting analysis were conducted to determine whether FNDC4 inhibited osteoclast formation via NF-κB signaling in RAW 264.7 cells. Furthermore, to identify gene signatures in FNDC4-treated BMMs and to use these to elucidate the underlying molecular mechanisms during osteoclast formation, we adopted a bioinformatics approach by downloading the GSE76172 gene expression profiling dataset from the Gene Expression Omnibus (GEO) database. FNDC4 inhibited RANKL-induced osteoclastogenesis and mature osteoclast resorptive function in a dose-dependent manner. Results of NF-κB luciferase assay suggested that FNDC4 could significantly suppress the RANKL-induced NF-κB transcriptional activity. Based on the protein-protein interaction network, CXCL10 was identified as the differentially expressed gene with the highest connectivity degree (degree = 23); it was drastically downregulated in the presence of FNDC4, but supplementation of CXCL10 (10 ng/mL) partially ameliorated the FNDC4-induced inhibition of osteoclast formation. Taken together, we speculated that FNDC4 could suppress osteoclast formation via NF-κB pathway and downregulation of CXCL10.
Figures

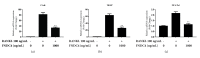


References
-
- Udagawa N., Takahashi N., Akatsu T., et al. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proceedings of the National Acadamy of Sciences of the United States of America. 1990;87(18):7260–7264. doi: 10.1073/pnas.87.18.7260. - DOI - PMC - PubMed
-
- Gamble C. L. Osteoporosis: drug and nondrug therapies for the patient at risk. Journal of the American Geriatrics Society. 1995;50(7):24–26. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

