Pneumococcal Conjugate Vaccine Safety in Elderly Adults
- PMID: 29977960
- PMCID: PMC6016414
- DOI: 10.1093/ofid/ofy100
Pneumococcal Conjugate Vaccine Safety in Elderly Adults
Abstract
Background: The 13-valent pneumococcal conjugate vaccine (PCV13) and the 23-valent pneumococcal polysaccharide vaccine (PPSV23) were both recommended to adults aged ≥65 years. The study examines adults ≥65 years for risk of adverse events (AEs) requiring medical attention following vaccination with PCV13 as compared with vaccination with PPSV23, a long-standing vaccine with a satisfactory safety profile.
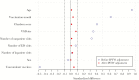
Methods: The cohort study included 6 Vaccine Safety Datalink sites. The exposed person-time included follow-up time of the first PCV13 received by subjects age ≥65 years from January 1 to August 15, 2015. The comparator person-time included follow-up time after the first PPSV23 received by subjects of the same age during Janaury 1 to August 15 of each year of 2011-2015. The prespecified AEs included cardiovascular events, Bell's palsy, Guillain-Barré syndrome, syncope, erythema multiforme, thrombocytopenia, cellulitis and infection, allergic reaction, and anaphylaxis. Inverse probability of treatment weighting-adjusted Poisson regression models was used to estimate the relative risk (RR) of each AE.
Results: A total of 313 136 doses of PCV13 and 232 591 doses of PPSV23 were included. The adjusted RRs comparing the incidence of AEs following PCV13 vs PPSV23 were all <1, except for anaphylaxis, which was insignificant with an RR of 1.32 (95% confidence interval, 0.30-5.79). Only 1 patient who received PCV13 and 4 other vaccines concomitantly was confirmed by medical chart review as having experienced anaphylaxis after vaccination.
Conclusions: These data do not support an increased rate of adverse events following PCV13 administration in elders compared with PPSV23 and should provide reassurance regarding continued use of PCV13.
Keywords: adult vaccination; adverse events; pneumococcal conjugate vaccine.
Figures
References
-
- Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect 2014; 20(Suppl 5):45–51. - PubMed
-
- Musher DM, Manof SB, Liss C et al. . Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Infect Dis 2010; 201:516–24. - PubMed
-
- Sankilampi U, Honkanen PO, Bloigu A, Leinonen M. Persistence of antibodies to pneumococcal capsular polysaccharide vaccine in the elderly. J Infect Dis 1997; 176:1100–4. - PubMed
-
- Metersky ML, Dransfield MT, Jackson LA. Determining the optimal pneumococcal vaccination strategy for adults: is there a role for the pneumococcal conjugate vaccine?Chest 2010; 138:486–90. - PubMed
-
- Jackson L, Neuzil K. Pneumococcal polysaccharide vaccines. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 5th ed Philadelphia, PA: Saunders, Elsevier;2008:570–604.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases