Exploration of the Main Sites for the Transformation of Normal Prion Protein (PrPC) into Pathogenic Prion Protein (PrPsc)
- PMID: 29978050
- PMCID: PMC5894410
- DOI: 10.1515/jvetres-2017-0002
Exploration of the Main Sites for the Transformation of Normal Prion Protein (PrPC) into Pathogenic Prion Protein (PrPsc)
Abstract
Introduction: The functions and mechanisms of prion proteins (PrPC) are currently unknown, but most experts believe that deformed or pathogenic prion proteins (PrPSc) originate from PrPC, and that there may be plural main sites for the conversion of normal PrPC into PrPSc. In order to better understand the mechanism of PrPC transformation to PrPSc, the most important step is to determine the replacement or substitution site.
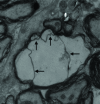
Material and methods: BALB/c mice were challenged with prion RML strain and from 90 days post-challenge (dpc) mice were sacrificed weekly until all of them had been at 160 dpc. The ultra-structure and pathological changes of the brain of experimental mice were observed and recorded by transmission electron microscopy.
Results: There were a large number of pathogen-like particles aggregated in the myelin sheath of the brain nerves, followed by delamination, hyperplasia, swelling, disintegration, phagocytic vacuolation, and other pathological lesions in the myelin sheath. The aggregated particles did not overflow from the myelin in unstained samples. The phenomenon of particle aggregation persisted all through the disease course, and was the earliest observed pathological change.
Conclusion: It was deduced that the myelin sheath and lipid rafts in brain nerves, including axons and dendrites, were the main sites for the conversion of PrPC to PrPSc, and the PrPSc should be formed directly by the conversion of protein conformation without the involvement of nucleic acids.
Keywords: brain; mice; myelin sheath; particle aggregation; prion proteins.
Conflict of interest statement
Conflict of Interests Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Figures











Similar articles
-
Prion Protein Devoid of the Octapeptide Repeat Region Delays Bovine Spongiform Encephalopathy Pathogenesis in Mice.J Virol. 2017 Dec 14;92(1):e01368-17. doi: 10.1128/JVI.01368-17. Print 2018 Jan 1. J Virol. 2017. PMID: 29046443 Free PMC article.
-
Strain-Dependent Prion Infection in Mice Expressing Prion Protein with Deletion of Central Residues 91-106.Int J Mol Sci. 2020 Oct 1;21(19):7260. doi: 10.3390/ijms21197260. Int J Mol Sci. 2020. PMID: 33019549 Free PMC article.
-
Prion protein functions and dysfunction in prion diseases.Curr Med Chem. 2009;16(3):380-9. doi: 10.2174/092986709787002673. Curr Med Chem. 2009. PMID: 19149584 Review.
-
The protease-sensitive N-terminal polybasic region of prion protein modulates its conversion to the pathogenic prion conformer.J Biol Chem. 2021 Nov;297(5):101344. doi: 10.1016/j.jbc.2021.101344. Epub 2021 Oct 25. J Biol Chem. 2021. PMID: 34710372 Free PMC article.
-
Virus Infection, Genetic Mutations, and Prion Infection in Prion Protein Conversion.Int J Mol Sci. 2021 Nov 18;22(22):12439. doi: 10.3390/ijms222212439. Int J Mol Sci. 2021. PMID: 34830321 Free PMC article. Review.
Cited by
-
Dysbiosis of the gut microbiota and its effect on α-synuclein and prion protein misfolding: consequences for neurodegeneration.Front Cell Infect Microbiol. 2024 Feb 16;14:1348279. doi: 10.3389/fcimb.2024.1348279. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38435303 Free PMC article. Review.
References
-
- Aguzzi A., Calella A.M.. Prions: protein aggregation and infectious diseases. Physiol Rev. 2009;89:1105–1152. - PubMed
-
- Bueler H., Fischer M., Lang Y., Bluethmann H., Lipp H.P., DeArmond S.J., Prusiner S.B., Aguet M., Weissmann C.. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–582. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
