Prevalence of Germline Mutations in Cancer Susceptibility Genes in Patients With Advanced Renal Cell Carcinoma
- PMID: 29978187
- PMCID: PMC6584283
- DOI: 10.1001/jamaoncol.2018.1986
Prevalence of Germline Mutations in Cancer Susceptibility Genes in Patients With Advanced Renal Cell Carcinoma
Abstract
Importance: Identification of patients with hereditary renal cell carcinoma (RCC) is important for cancer screening and, in patients with advanced disease, for guiding treatment. The prevalence of cancer-related germline mutations in patients with advanced RCC and the phenotypes associated with some rare mutations are unknown.
Objectives: To examine the prevalence of germline mutations in both known RCC predisposition genes and other cancer-associated genes and to identify clinical and pathologic factors associated with germline mutations.
Design, setting, and participants: In this cohort study conducted from October 1, 2015, to July 31, 2017, 254 of 267 patients with advanced (American Joint Committee on Cancer stage III or IV) RCC who were seen in medical oncology or urology clinics agreed to germline sequencing and disclosure of results under an institutional protocol of matched tumor-germline DNA sequencing.
Main outcomes and measures: Mutation prevalence and spectrum in patients with advanced RCC were determined. Clinical characteristics were assessed by mutation status.
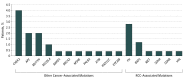
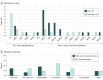
Results: Of the 254 patients (median age [range], 56 [13-79] years; 179 [70.5%] male; 211 [83.1%] non-Hispanic white), germline mutations were identified in 41 (16.1%); 14 (5.5%) had mutations in syndromic RCC-associated genes (7 in FH, 3 in BAP1, and 1 each in VHL, MET, SDHA, and SDHB). The most frequent mutations were CHEK2 (n = 9) and FH (n = 7). Of genes not previously associated with RCC risk, CHEK2 was overrepresented in patients compared with the general population, with an odds ratio of RCC of 3.0 (95% CI, 1.3-5.8; P = .003). Patients with non-clear cell RCC were significantly more likely to have an RCC-associated gene mutation (9 [11.7%] of 74 vs 3 [1.7%] of 177; P = .001), and 8 (10.0%) had a mutation in a gene that could guide therapy. Of patients with mutations in RCC-associated genes, 5 (35.7%) failed to meet current clinical guidelines for genetic testing.
Conclusions and relevance: Of patients with non-clear cell RCC, more than 20% had a germline mutation, of which half had the potential to direct systemic therapy. Current referral criteria for genetic testing did not identify a substantial portion of patients with mutations, supporting the role of a more inclusive sequencing approach.
Conflict of interest statement
Figures

Comment in
-
Broadening the View of Germline Mutations in Kidney Cancer.JAMA Oncol. 2018 Sep 1;4(9):1235-1236. doi: 10.1001/jamaoncol.2018.2015. JAMA Oncol. 2018. PMID: 29978199 No abstract available.
-
CHEK2 Alleles Predispose to Renal Cancer in Poland-In Reply.JAMA Oncol. 2019 Apr 1;5(4):576-577. doi: 10.1001/jamaoncol.2019.0025. JAMA Oncol. 2019. PMID: 30816934 No abstract available.
-
CHEK2 Alleles Predispose to Renal Cancer in Poland.JAMA Oncol. 2019 Apr 1;5(4):576. doi: 10.1001/jamaoncol.2019.0022. JAMA Oncol. 2019. PMID: 30816943 No abstract available.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. - PubMed
-
- Stephenson AJ, Chetner MP, Rourke K, et al. . Guidelines for the surveillance of localized renal cell carcinoma based on the patterns of relapse after nephrectomy. J Urol. 2004;172(1):58-62. - PubMed
-
- Dabestani S, Thorstenson A, Lindblad P, Harmenberg U, Ljungberg B, Lundstam S. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World J Urol. 2016;34(8):1081-1086. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

