Glycal Metallanitrenes for 2-Amino Sugar Synthesis: Amidoglycosylation of Gulal-, Allal-, Glucal-, and Galactal 3-Carbamates
- PMID: 29979042
- PMCID: PMC6662188
- DOI: 10.1021/acs.joc.8b00893
Glycal Metallanitrenes for 2-Amino Sugar Synthesis: Amidoglycosylation of Gulal-, Allal-, Glucal-, and Galactal 3-Carbamates
Abstract
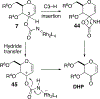
The rhodium(II)-catalyzed oxidative cyclization of glycal 3-carbamates with in situ incorporation of an alcohol nucleophile at the anomeric position provides access to a range of 2-amino sugars having 1,2-trans-2,3-cis stereochemistry, a structural motif present in compounds of medicinal and biological significance such as the streptothricin group of antibiotics and the Chitinase inhibitor allosamidin. All of the diastereomeric d-glycal 3-carbamates have been investigated, revealing significant differences in anomeric stereoselectivity depending on substrate stereochemistry and protecting groups. In addition, some substrates were prone to forming C3-oxidized dihydropyranone byproducts under the reaction conditions. Allal- and gulal 3-carbamates provided uniformly high stereo- and chemoselectivity, while for glucal substrates, acyclic, electron-withdrawing protecting groups at the 4 O and 6 O positions were required. Galactal 3-carbamates have been the most challenging substrates; formation of their amidoglycosylation products is most effective with an electron-withdrawing 6 O-Ts substituent and a sterically demanding 4 O-TBS group. These results suggest a mechanism whereby conformational and electronic factors determine the partitioning of an intermediate acyl nitrenoid between alkene addition, leading to amidoglycosylation, and C3-H insertion, providing the dihydropyranone byproduct. Along the amidoglycosylation pathway, high anomeric selectivity results when a glycosyl aziridine intermediate is favored over an aziridine-opened oxocarbenium donor.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
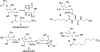
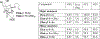
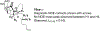
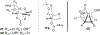
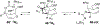


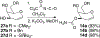

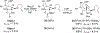

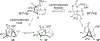
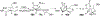
References
-
- Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, Eds. Essentials of Glycobiology, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, 2009. - PubMed
- Galonić DP; Gin DY Chemical Glycosylation in the Synthesis of Glycoconjugate Antitumour Vaccines. Nature 2007, 446, 1000–1007. - PMC - PubMed
- Seeberger PH; Werz DB Synthesis and Medical Applications of Oligosaccharides. Nature 2007, 446, 1046–1051. - PubMed
-
- Magano J Synthetic Approaches to the Neuraminidase Inhibitors Zanamivir (Relenza) and Oseltamivir Phosphate (Tamiflu) for the Treatment of Influenza. Chem. Rev 2009, 109, 4398–4438. - PubMed
- Silva JG; Carvalho I New Insights into Aminoglycoside Antibiotics and Derivatives. Curr. Med. Chem 2007, 14, 1101–1119. - PubMed
- Moscona A Neuraminidase Inhibitors for Influenza. N. Engl. J. Med 2005, 353, 1363–73. - PubMed
- Magnet S; Blanchard JS Molecular Insights into Aminoglycoside Action and Resistance. Chem. Rev 2005, 105, 477–497. - PubMed
-
- Skarbek K; Milewska MJ Biosynthetic and Synthetic Access to Amino Sugars. Carbohydr. Res 2016, 434, 44–71. - PubMed
- Adibekian A; Timmer MSM; Stallforth P; van Rijn J; Werz DB; Seeberger PH Stereocontrolled Synthesis of Fully Functionalized D-Glucosamine Monosaccharides via a Domino Nitro-Michael/Henry Reaction. Chem. Commun 2008, 3549–3551. - PubMed
- Bongat AFG; Demchenko AV Recent Trends in the Synthesis of O-Glycosides of 2-Amino-2-Deoxysugars. Carbohydr. Res 2007, 342, 374–406. - PubMed
-
- Kusumoto S; Kambayashi Y; Imaoka S; Shima K; Shiba T Biosynthesis of Natural Products Containing β-Amino Acids. J. Antibiot 1982, 35, 925–927. - PubMed
- Gan M; Zheng X; Liu Y; Guan Y; Xiao C Three New 12-Carbamoylated Streptothricins from Streptomyces sp. I08A 1776. Bioorg. Med. Chem. Lett 2012, 22, 6151– 6154. - PubMed
-
- Berecibar A; Grandjean C; Siriwardena A Synthesis and Biological Activity of Natural Aminocyclopentitol Glycosidase Inhibitors: Mannostatins, Trehazolin, Allosamidins, and Their Analogues. Chem. Rev 1999, 99, 779–844. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

