An automated high-resolution in vivo screen in zebrafish to identify chemical regulators of myelination
- PMID: 29979149
- PMCID: PMC6056238
- DOI: 10.7554/eLife.35136
An automated high-resolution in vivo screen in zebrafish to identify chemical regulators of myelination
Abstract
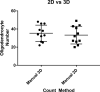
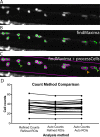
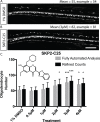
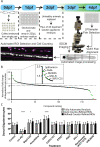
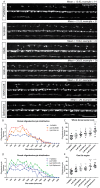
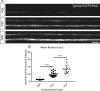
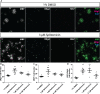
Myelinating oligodendrocytes are essential for central nervous system (CNS) formation and function. Their disruption is implicated in numerous neurodevelopmental, neuropsychiatric and neurodegenerative disorders. However, recent studies have indicated that oligodendrocytes may be tractable for treatment of disease. In recent years, zebrafish have become well established for the study of myelinating oligodendrocyte biology and drug discovery in vivo. Here, by automating the delivery of zebrafish larvae to a spinning disk confocal microscope, we were able to automate high-resolution imaging of myelinating oligodendrocytes in vivo. From there, we developed an image analysis pipeline that facilitated a screen of compounds with epigenetic and post-translational targets for their effects on regulating myelinating oligodendrocyte number. This screen identified novel compounds that strongly promote myelinating oligodendrocyte formation in vivo. Our imaging platform and analysis pipeline is flexible and can be employed for high-resolution imaging-based screens of broad interest using zebrafish.
Keywords: VAST; automated chemical screening; myelination; neuroscience; oligodendrocyte; zebrafish.
© 2018, Early et al.
Conflict of interest statement
JE, MS, DL No competing interests declared, KC funded by a collaborative grant from Biogen for part of the period of this project, JW Jill M Williamson: funded by a collaborative grant from Biogen for part of the period of this project, HK was employed by Biogen at the time of the study, MM Marc Muskavitch: was employed by Biogen at the time of the study
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

