Eyeless/Pax6 initiates eye formation non-autonomously from the peripodial epithelium
- PMID: 29980566
- PMCID: PMC6110151
- DOI: 10.1242/dev.163329
Eyeless/Pax6 initiates eye formation non-autonomously from the peripodial epithelium
Abstract
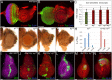
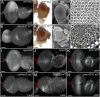
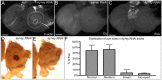
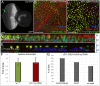
The transcription factor Pax6 is considered the master control gene for eye formation because (1) it is present within the genomes and retina/lens of all animals with a visual system; (2) severe retinal defects accompany its loss; (3) Pax6 genes have the ability to substitute for one another across the animal kingdom; and (4) Pax6 genes are capable of inducing ectopic eye/lens in flies and mammals. Many roles of Pax6 were first elucidated in Drosophila through studies of the gene eyeless (ey), which controls both growth of the entire eye-antennal imaginal disc and fate specification of the eye. We show that Ey also plays a surprising role within cells of the peripodial epithelium to control pattern formation. It regulates the expression of decapentaplegic (dpp), which is required for initiation of the morphogenetic furrow in the eye itself. Loss of Ey within the peripodial epithelium leads to the loss of dpp expression within the eye, failure of the furrow to initiate, and abrogation of retinal development. These findings reveal an unexpected mechanism for how Pax6 controls eye development in Drosophila.
Keywords: Dorsal-ventral patterning; Drosophila; Eye; Eyeless; Morphogenetic furrow; Pax6; Peripodial epithelium; Retina; Twin of Eyeless.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures




References
-
- Baron A. L. (1935). Facet number in Drosophila melanogaster as influenced by certain genetic and environmental factors. J. Exp. Zool. 70, 461-490. 10.1002/jez.1400700307 - DOI
-
- Blackman R. K., Sanicola M., Raftery L. A., Gillevet T. and Gelbart W. M. (1991). An extensive 3′ cis-regulatory region directs the imaginal disk expression of decapentaplegic, a member of the TGF-b family in Drosophila. Development 111, 657-665. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

