Biologic refractory disease in rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis
- PMID: 29980575
- PMCID: PMC6161665
- DOI: 10.1136/annrheumdis-2018-213378
Biologic refractory disease in rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis
Abstract
Objectives: Biologic disease-modifying antirheumatic drugs (bDMARDs) have revolutionised treatment and outcomes for rheumatoid arthritis (RA). The expanding repertoire allows the option of switching bDMARD if current treatment is not effective. For some patients, even after switching, disease control remains elusive. This analysis aims to quantify the frequency of, and identify factors associated with, bDMARD refractory disease.
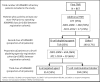
Methods: Patients with RA starting first-line tumour necrosis factor inhibitor in the British Society for Rheumatology Biologics Register for RA from 2001 to 2014 were included. We defined patients as bDMARD refractory on the date they started their third class of bDMARD. Follow-up was censored at last follow-up date, 30 November 2016, or death, whichever came first. Switching patterns and stop reasons of bDMARDs were investigated. Cox regression identified baseline clinical factors associated with refractory disease. Multiple imputation of missing baseline data was used.
Results: 867 of 13 502 (6%) patients were bDMARD refractory; median time to third bDMARD class of 8 years. In the multivariable analysis, baseline factors associated with bDMARD refractory disease included patients registered more recently, women, younger age, shorter disease duration, higher patient global assessment, higher Health Assessment Questionnaire score, current smokers, obesity and greater social deprivation.
Conclusions: This first national study has identified the frequency of bDMARD refractory disease to be at least 6% of patients who have ever received bDMARDs. As the choice of bDMARDs increases, patients are cycling through bDMARDs quicker. The aetiopathogenesis of bDMARD refractory disease requires further investigation. Focusing resources, such as nursing support, on these patients may help them achieve more stable, controlled disease.
Keywords: dmards (biologic); epidemiology; rheumatoid arthritis.
© Author(s) (or their employer(s)) 2018. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: MHB has received grants from Pfizer Ltd and Roche Pharmaceuticals, as well as expert advice and honoraria from Abbvie, Astra-Zeneca, BMS, Lilly, Roche, Sandoz and UCB. JDI has received research grants from Pfizer Ltd and Roche Pharmaceuticals, as well as honoraria/fees from Abbvie, Roche, Pfizer, Janssen and BMS.
Figures

References
-
- NICE. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for rheumatoid arthritis not previously treated with DMARDs or after conventional DMARDs only have failed: National Institue for Health and Care Excellence. 2016. Report No: Technology Appraisal Guidance: TA375.
-
- NICE. TA195: Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a TNF inhibitor: National Institue for Health and Care Excellence. 2010. Report No: Technology Appraisal Guidance: TA195.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

