Foxp3+ T reg cells control psoriasiform inflammation by restraining an IFN-I-driven CD8+ T cell response
- PMID: 29980582
- PMCID: PMC6080913
- DOI: 10.1084/jem.20172094
Foxp3+ T reg cells control psoriasiform inflammation by restraining an IFN-I-driven CD8+ T cell response
Abstract
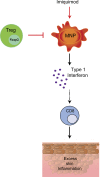
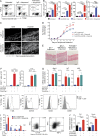
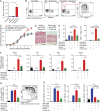
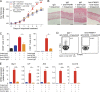
Psoriasis is a complex inflammatory skin disease affecting ∼3% of the population worldwide. Although type I interferons (IFN-I) are thought to be involved in its pathogenesis, the details of this relationship remain elusive. Here we show that in a murine model of imiquimod-driven psoriatic skin inflammation, Foxp3+ regulatory T cells (T reg cells) control inflammation severity by restraining IFN-I. Depletion of T reg cells induces IFN-I and IFN-stimulated gene expression, and leads to accumulation of CD8+ T cells in lesional skin. Mononuclear phagocytes (MNPs) were the source of IFN-I, and their depletion reversed the effect of T reg cell depletion. Blockade of IFN-I signaling abolished CD8+ T cell infiltration and excess inflammation in the skin of T reg cell-depleted mice. Depletion of CD8+ T cells attenuated pathology, confirming their role as critical effector cells downstream of IFN-I. Our results describe an unexpected role for T reg cells in restraint of an MNP-IFN-I-driven CD8+ T cell response during psoriasiform skin inflammation. These findings highlight a pathway with potential relevance for the treatment of early-stage disease.
© 2018 Stockenhuber et al.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

