Thermal proteome profiling in bacteria: probing protein state in vivo
- PMID: 29980614
- PMCID: PMC6056769
- DOI: 10.15252/msb.20188242
Thermal proteome profiling in bacteria: probing protein state in vivo
Abstract
Increasing antibiotic resistance urges for new technologies for studying microbes and antimicrobial mechanism of action. We adapted thermal proteome profiling (TPP) to probe the thermostability of Escherichia coli proteins in vivoE. coli had a more thermostable proteome than human cells, with protein thermostability depending on subcellular location-forming a high-to-low gradient from the cell surface to the cytoplasm. While subunits of protein complexes residing in one compartment melted similarly, protein complexes spanning compartments often had their subunits melting in a location-wise manner. Monitoring the E. coli meltome and proteome at different growth phases captured changes in metabolism. Cells lacking TolC, a component of multiple efflux pumps, exhibited major physiological changes, including differential thermostability and levels of its interaction partners, signaling cascades, and periplasmic quality control. Finally, we combined in vitro and in vivo TPP to identify targets of known antimicrobial drugs and to map their downstream effects. In conclusion, we demonstrate that TPP can be used in bacteria to probe protein complex architecture, metabolic pathways, and intracellular drug target engagement.
Keywords: Escherichia coli; metabolic pathways; protein complexes; target engagement; thermal proteome profiling.
© 2018 The Authors. Published under the terms of the CC BY 4.0 license.
Figures

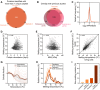
Thermal proteome profiling protocol overview. After cells are grown to a specified optical density (OD578), aliquots are heated to a range of temperatures, lysed, and the remaining soluble fraction of the proteome is collected. Mass spectrometry‐based proteomics (using tandem mass tags, TMT) is then used to quantify the amount of protein at each condition, and melting curves are plotted for each protein.
Melting curves for E. coli proteins. The average melting curve for each cellular compartment is shown.
Distribution of melting temperatures (T m) of the E. coli and the human proteomes.
Distribution of melting temperatures (T m) of the E. coli and the human proteomes according to selected gene ontology terms. Line represents the median, box represents the interquartile range, and whiskers of the box plots represent the 5th and 95th percentiles.
Distribution of melting temperatures (T m) of the E. coli proteome according to their cellular compartment. Box plots are plotted as panel (D).
Correlation of melting points in lysate determined by TPP (this study) with melting points determined by limited proteolysis coupled to mass spectrometry (Leuenberger et al, 2017). For the results from Leuenberger et al (2017), the median melting point of the reported peptides for each protein was used. Only proteins with at least two identified peptides were compared. Red dots represent ribosomal proteins, which generally appear less thermostable in TPP.
Correlation of melting points in lysate determined by TPP upon addition of 10 mM MgCl2 (this study) with melting points determined by limited proteolysis coupled to mass spectrometry (Leuenberger et al, 2017). For the results from Leuenberger et al (2017), the median melting point of the reported peptides for each protein was used. Only proteins with at least two identified peptides were compared. Red dots represent ribosomal proteins.
- A
Reproducibility of identified proteins in each replicate of E. coli meltome analysis.
- B
Overlap of identified proteins with previously published proteomics datasets obtained from E. coli.
- C
Distribution of differences between protein abundance after being extracted with NP‐40 or with SDS.
- D, E
Correlation of melting point with (D) protein abundance (r = 0.06, P = 0.015, as measured by the top3 intensity corresponding to the lowest temperature) and (E) molecular weight (r = −0.08, P = 0.0009).
- F
Correlation of melting point in living cells with melting point in lysate—both from TPP (r = 0.82, P < 0.0001).
- G
Melting curves for E. coli outer membrane proteins. The average melting curve for each class of outer membrane proteins is shown.
- H
Distribution of melting temperatures (T m) of the E. coli proteome according to their cellular compartment.
- I
Fraction of proteins with T m > 87°C (the highest temperature tested) in each cellular compartment.
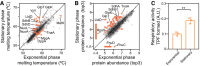
Melting temperatures (T m) of proteins in exponential and transition to stationary growth phases. Proteins highlighted in orange indicate significantly different melting behavior.
Protein abundance in exponential and transition to stationary growth phases, as measured by the top3 intensity corresponding to the lowest temperature (see “Materials and Methods”). Proteins highlighted in orange indicate significantly different levels. Proteins were considered not detectable (n.d.) in one condition, if absent in three replicates in that condition, but detectable by at least three unique peptides in at least two replicates in the other condition.
Respiratory activity in exponential and stationary cells determined as the conversion of triphenyltetrazolium chloride to triphenylformazan during the same time and normalized by OD (˜number of cells). n = 3; error bars represent standard deviation; **P < 0.01, Student's t‐test.
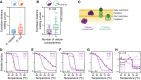
- A
Melting behavior of protein complexes from human and Escherichia coli was measured by the average Euclidean distance between the melting curves of proteins from each complex. Line represents the median, box represents the interquartile range, and whiskers of the box plots represent the 10th and 90th percentiles. Pie charts represent the fraction of protein complexes that melt coherently (compared with a distribution of 10,000 random complexes; P < 0.05).
- B
Comparison of the melting behavior of protein complexes located in a single cellular compartment or in multiple compartments. Line represents the median, box represents the interquartile range, and whiskers of the box plots represent the 10th and 90th percentiles. Pie charts represent the fraction of protein complexes that melt coherently (compared with a distribution of 10,000 random complexes; P < 0.05).
- C
Schematic representation of complexes located in a single cellular compartment or in multiple compartments.
- D–H
Melting curves for examples of non‐co‐melting complexes located in the same cellular compartment: (D) ClpP protease complex, (E) Ruv DNA repair complex, (F) Uvr DNA repair complex, (G) Suf Fe‐S biogenesis complex, and (H) Bam outer membrane porin assembly complex. P indicates the probability that the complex melts coherently (compared with a distribution of 10,000 random complexes).

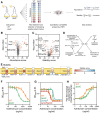
- A
Two‐dimensional thermal proteome profiling (2D‐TPP) protocol overview. Wild‐type (WT) and tolC knockout strain (ΔtolC) were grown and prepared in a similar manner to what is described in Fig 1A. For each protein, abundance and stability scores were calculated.
- B, C
Volcano plots for abundance (B) and stability (C) scores for each identified protein in ΔtolC compared to WT (TolC signal is detected at noise level in the ΔtolC strain, due to its presence in WT and TMT quantification rarely producing missing values). Proteins highlighted in orange show significant changes [false discovery rate (FDR) P < 0.05 and absolute score > 10].
- D
Proposed mechanism for abundance and stability hits of ΔtolC.
- E
Schematic representation of TolC complexes and the stability scores of their members. YbhG is a member of a putative efflux transporter. *False discovery rate (FDR) P < 0.05 and absolute score > 10. n.d. not detected.
- F, G
Cell growth (as measured by OD595) after 8 h in the presence of azithromycin (F) or aztreonam (G) in WT, ΔtolC, ΔompF, and ΔmicFΔtolC cells (n = 4; error bars represent standard deviation).
- H
Target engagement affinity of aztreonam in WT and ΔtolC cells, measured by thermal proteome profiling–compound concentration range (TPP‐CCR). Stabilization of the main known target of aztreonam (FtsI) is shown.

- A, B
Interaction network of TolC color‐coded by the (A) abundance score or (B) stability score. Network was obtained from STRING database by querying only the statistically significant hits (in both abundance and stability) and their interactions with a confidence score of > 0.4.
- C
Growth of ΔsurAΔtolC in the absence or presence of 1 mM MgSO4 and 0.1 mM CaCl2 in LB medium containing 4 mM sodium citrate with or without 30 μg/ml kanamycin. Viability was determined by spotting serial dilutions (100−10−6) of overnight cultures.
- D
Cell growth (as measured by OD595) in LB supplemented with 600 mM NaCl of WT, ΔtolC, ΔsurA, and ΔsurAΔtolC from a starting culture at OD595 = 0.2.
- E
Growth of WT, ΔtolC, ΔsurA, and ΔsurAΔtolC in increasing concentrations of NaCl in LB. Viability was determined by spotting serial dilutions (100–10−6) of overnight cultures.
- F
Cell growth (as measured by OD595) after 8 h in the presence of aztreonam in WT, ΔtolC, ΔompF and ΔompF ΔtolC cells (n = 4; error bars represent standard deviation).
- G
Cell growth (as measured by OD595) after 8 h in the presence of aztreonam in WT, ΔtolC, ΔmicF, and ΔmicF ΔtolC cells (n = 4; error bars represent standard deviation).
- H
Target engagement affinity of aztreonam in WT and ΔtolC cells, measured by thermal proteome profiling compound concentration range (TPP‐CCR). Stabilization of the secondary known target of aztreonam (MrcA) is shown.

2D‐TPP protocol overview. After treatment with different concentrations of antibiotics, the cells were prepared in a similar manner to what is described in Fig 1A. For each protein and temperature, the signal intensity was normalized to the vehicle control.
Heatmaps for targets of ampicillin in living cells, with coloring according to panel (A). *FDR controlled at 1% using a bootstrapped permutation approach.
Number of stabilized or destabilized proteins in lysate and living cells after treatment with ampicillin.
Example of top gene ontology terms enriched in proteins affected in living cells after treatment with ampicillin.
Heatmaps for targets of ciprofloxacin in living cells, with coloring according to what is described in panel (A). *FDR controlled at 1% using a bootstrapped permutation approach.
Schematic representation of SOS response. LexA binds to promoters of SOS response genes and represses their transcription. Treatment with ciprofloxacin induces single‐ and double‐stranded DNA breaks that recruit RecA to DNA, causing auto‐cleavage of LexA and expression of SOS response genes (e.g., yebG; Andersson & Hughes, 2014).

- A, B
Heatmaps of effects on thermostability of proteins after treatment with (A) ampicillin and (B) ciprofloxacin in lysate, with coloring according to what is described in Fig 5A. *FDR controlled at 1% using a bootstrapped permutation approach.
Similar articles
-
Integrated changes in thermal stability and proteome abundance during altered nutrient states in Escherichia coli and human cells.Proteomics. 2022 Oct;22(19-20):e2100254. doi: 10.1002/pmic.202100254. Epub 2022 Sep 16. Proteomics. 2022. PMID: 36082775 Free PMC article.
-
The functional proteome landscape of Escherichia coli.Nature. 2020 Dec;588(7838):473-478. doi: 10.1038/s41586-020-3002-5. Epub 2020 Dec 9. Nature. 2020. PMID: 33299184 Free PMC article.
-
Thermal Proteome Profiling and Meltome Analysis of a Thermophilic Bacterial Strain, Geobacillus thermoleovorans ARTRW1: Toward Industrial Applications.OMICS. 2020 Dec;24(12):756-765. doi: 10.1089/omi.2020.0115. Epub 2020 Oct 20. OMICS. 2020. PMID: 33085568
-
Thermal proteome profiling for interrogating protein interactions.Mol Syst Biol. 2020 Mar;16(3):e9232. doi: 10.15252/msb.20199232. Mol Syst Biol. 2020. PMID: 32133759 Free PMC article. Review.
-
Proteome of the Escherichia coli envelope and technological challenges in membrane proteome analysis.Biochim Biophys Acta. 2008 Sep;1778(9):1698-713. doi: 10.1016/j.bbamem.2007.07.020. Epub 2007 Aug 11. Biochim Biophys Acta. 2008. PMID: 17904518 Review.
Cited by
-
Recent progress in mass spectrometry-based strategies for elucidating protein-protein interactions.Cell Mol Life Sci. 2021 Jul;78(13):5325-5339. doi: 10.1007/s00018-021-03856-0. Epub 2021 May 27. Cell Mol Life Sci. 2021. PMID: 34046695 Free PMC article. Review.
-
A small-molecule inhibitor of BamA impervious to efflux and the outer membrane permeability barrier.Proc Natl Acad Sci U S A. 2019 Oct 22;116(43):21748-21757. doi: 10.1073/pnas.1912345116. Epub 2019 Oct 7. Proc Natl Acad Sci U S A. 2019. PMID: 31591200 Free PMC article.
-
Outer membrane lipoprotein NlpI scaffolds peptidoglycan hydrolases within multi-enzyme complexes in Escherichia coli.EMBO J. 2020 Mar 2;39(5):e102246. doi: 10.15252/embj.2019102246. Epub 2020 Feb 3. EMBO J. 2020. PMID: 32009249 Free PMC article.
-
Human gut bacteria bioaccumulate per- and polyfluoroalkyl substances.Nat Microbiol. 2025 Jul;10(7):1630-1647. doi: 10.1038/s41564-025-02032-5. Epub 2025 Jul 1. Nat Microbiol. 2025. PMID: 40595288 Free PMC article.
-
The Cu(II) Reductase RclA Protects Escherichia coli against the Combination of Hypochlorous Acid and Intracellular Copper.mBio. 2020 Sep 29;11(5):e01905-20. doi: 10.1128/mBio.01905-20. mBio. 2020. PMID: 32994322 Free PMC article.
References
-
- Aebersold R, Mann M (2016) Mass‐spectrometric exploration of proteome structure and function. Nature 537: 347–355 - PubMed
-
- Andersson DI, Hughes D (2014) Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol 12: 465–478 - PubMed
-
- Babu M, Bundalovic‐Torma C, Calmettes C, Phanse S, Zhang Q, Jiang Y, Minic Z, Kim S, Mehla J, Gagarinova A, Rodionova I, Kumar A, Guo H, Kagan O, Pogoutse O, Aoki H, Deineko V, Caufield JH, Holtzapple E, Zhang Z et al (2017) Global landscape of cell envelope protein complexes in Escherichia coli . Nat Biotechnol 36: 103–112 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

