Lifespan and functionality of mycorrhizal fungal mycelium are uncoupled from host plant lifespan
- PMID: 29980700
- PMCID: PMC6035242
- DOI: 10.1038/s41598-018-28354-5
Lifespan and functionality of mycorrhizal fungal mycelium are uncoupled from host plant lifespan
Abstract
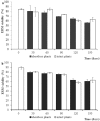
Arbuscular mycorrhizal fungi (AMF) are obligate symbionts, living in associations with the roots of most land plants. AMF produce wide networks of extraradical mycelium (ERM) of indeterminate length, spreading from host roots into the surrounding soil and establishing belowground interconnections among plants belonging to the same or to different taxa. Whether their lifespan and functionality are limited by host plant viability or can be extended beyond this limit is unknown. To address this issue, we performed time-course studies to investigate viability and functionality of ERM produced in an in vivo whole-plant system by Funneliformis mosseae and Rhizoglomus irregulare, after shoot detachment. Our data revealed that viability and functionality of F. mosseae and R. irregulare extraradical hyphae were uncoupled from host plant lifespan. Indeed, ERM spreading from roots of intact or shootless plants showed comparable levels of viability, similar structural traits and ability to establish mycorrhizal symbioses with new plants, as long as five months after shoot removal. Our findings expand the current knowledge on AMF biology and life cycle, providing data on ERM long-term survival in the soil of two Glomeracean species, functional to the prompt establishment of mycorrhizal symbioses and to the maintenance of soil biological fertility.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Mosse B. Observations on the extra-matrical mycelium of a vesicular-arbuscular endophyte. T. Br. Mycol. Soc. 1959;42:439–448. doi: 10.1016/S0007-1536(59)80044-9. - DOI
-
- Clark RB, Zeto SK. Mineral acquisition by arbuscular mycorrhizal plants. J. Plant Nutr. 2000;23:867–902. doi: 10.1080/01904160009382068. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

