Multiple liver insults synergize to accelerate experimental hepatocellular carcinoma
- PMID: 29980757
- PMCID: PMC6035229
- DOI: 10.1038/s41598-018-28486-8
Multiple liver insults synergize to accelerate experimental hepatocellular carcinoma
Abstract
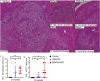
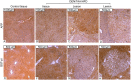
The urgent unmet need for hepatocellular carcinoma (HCC) therapies is addressed here by characterising a novel mouse model of HCC in the context of ongoing liver damage and overnutrition. Male C57Bl/6J mice were treated with diethylnitrosamine (DEN) and thioacetamide (TAA), and some were provided with an atherogenic high fat diet (HFD). Inflammation, steatosis, fibrosis, 87 genes, liver lesions and intratumoural leukocyte subsets were quantified up to 24 weeks of age. Adding HFD to DEN/TAA increased fibrosis, steatosis and inflammation, and the incidence of both HCC and non-HCC dysplastic lesions. All lesions contained α-SMA positive fibroblasts. Macrophage marker F4/80 was not significantly different between treatment groups, but the macrophage-associated genes Arg-1 and Cd47 were differentially expressed. Fibrosis, cancer and cell death associated genes were upregulated in DEN/TAA/HFD livers. Fewer Kupffer cells and plasmacytoid dendritic cells were in tumours compared to control liver. In conclusion, combining a hepatotoxin with an atherogenic diet produced more intrahepatic tumours, dysplastic lesions and fibrosis compared to hepatotoxin alone. This new HCC model provides a relatively rapid means of examining primary HCC and potential therapies in the context of multiple hepatotoxins including those derived from overnutrition.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

