TMEM43-S358L mutation enhances NF-κB-TGFβ signal cascade in arrhythmogenic right ventricular dysplasia/cardiomyopathy
- PMID: 29980933
- PMCID: PMC6340891
- DOI: 10.1007/s13238-018-0563-2
TMEM43-S358L mutation enhances NF-κB-TGFβ signal cascade in arrhythmogenic right ventricular dysplasia/cardiomyopathy
Abstract
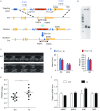
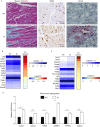
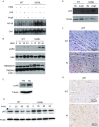
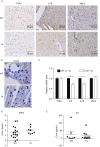
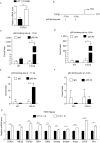
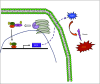
Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) is a genetic cardiac muscle disease that accounts for approximately 30% sudden cardiac death in young adults. The Ser358Leu mutation of transmembrane protein 43 (TMEM43) was commonly identified in the patients of highly lethal and fully penetrant ARVD subtype, ARVD5. Here, we generated TMEM43 S358L mouse to explore the underlying mechanism. This mouse strain showed the classic pathologies of ARVD patients, including structural abnormalities and cardiac fibrofatty. TMEM43 S358L mutation led to hyper-activated nuclear factor κB (NF-κB) activation in heart tissues and primary cardiomyocyte cells. Importantly, this hyper activation of NF-κB directly drove the expression of pro-fibrotic gene, transforming growth factor beta (TGFβ1), and enhanced downstream signal, indicating that TMEM43 S358L mutation up-regulates NF-κB-TGFβ signal cascade during ARVD cardiac fibrosis. Our study partially reveals the regulatory mechanism of ARVD development.
Keywords: ARVD; NF-κB; TGFβ; TMEM43; fibrosis; knock-in mouse.
Figures
References
-
- Asimaki A, Tandri H, Duffy ER, Winterfield JR, Mackey-Bojack S, Picken MM, Cooper LT, Wilber DJ, Marcus FI, Basso C, et al. Altered desmosomal proteins in granulomatous myocarditis and potential pathogenic links to arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:743–752. doi: 10.1161/CIRCEP.111.964890. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

