Ketorolac for postoperative pain in children
- PMID: 29981164
- PMCID: PMC6513208
- DOI: 10.1002/14651858.CD012294.pub2
Ketorolac for postoperative pain in children
Abstract
Background: Children who undergo surgical procedures in ambulatory and inpatient settings are at risk of experiencing acute pain. Nonsteroidal anti-inflammatory drugs (NSAIDs) can reduce moderate to severe pain without many of the side effects associated with opioids. However, NSAIDs may cause bleeding, renal and gastrointestinal toxicity, and potentially delay wound and bone healing. Intravenous administration of ketorolac for postoperative pain in children has not been approved in many countries, but is routinely administered in clinical practise.
Objectives: To assess the efficacy and safety of ketorolac for postoperative pain in children.
Search methods: We searched the following databases, without language restrictions, to November 2017: CENTRAL (The Cochrane Library 2017, Issue 10); MEDLINE, Embase, and LILACS. We also checked clinical trials registers and reference lists of reviews, and retrieved articles for additional studies.
Selection criteria: We included randomised controlled trials that compared the analgesic efficacy of ketorolac (in any dose, administered via any route) with placebo or another active treatment, in treating postoperative pain in participants zero to 18 years of age following any type of surgery.
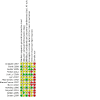
Data collection and analysis: We used standard methodological procedures expected by Cochrane. Two review authors independently considered trials for inclusion in the review, assessed risk of bias, and extracted data. We analyzed trials in two groups; ketorolac versus placebo, and ketorolac versus opioid. However, we performed limited pooled analyses. We assessed the overall quality of the evidence for each outcome using GRADE, and created a 'Summary of findings' table.
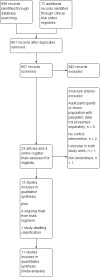
Main results: We included 13 studies, involving 920 randomised participants. There was considerable heterogeneity among study designs, including the comparator arms (placebo, opioid, another NSAID, or a different regimen of ketorolac), dosing regimens (routes and timing of administration, single versus multiple dose), outcome assessment methods, and types of surgery. Mean study population ages ranged from 356 days to 13.9 years. The majority of studies chose a dose of either 0.5 mg/kg (as a single or multiple dose regimen) or 1 mg/kg (single dose with 0.5 mg/kg for any subsequent doses). One study administered interventions intraoperatively; the remainder administered interventions postoperatively, often after the participant reported moderate to severe pain.There were insufficient data to perform meta-analysis for either of our primary outcomes: participants with at least 50% pain relief; or mean postoperative pain intensity. Four studies individually reported statistically significant reductions in pain intensity when comparing ketorolac with placebo, but the studies were small and had various risks of bias, primarily due to incomplete outcome data and small sample sizes.We found limited data available for the secondary outcomes of participants requiring rescue medication and opioid consumption. For the former, we saw no clear difference between ketorolac and placebo; 74 of 135 (55%) participants receiving ketorolac required rescue analgesia in the post-anaesthesia care unit (PACU) versus 81 of 127 (64%) receiving placebo (relative risk (RR) 0.85, 95% confidence interval (CI) 0.71 to 1.00, P = 0.05; 4 studies, 262 participants). For opioid consumption in the PACU, we saw no clear difference between ketorolac and placebo (P = 0.61). For the time period zero to four hours after administration of the interventions, participants receiving ketorolac received 1.58 mg less intravenous morphine equivalents than those receiving placebo (95% CI -2.58 mg to -0.57 mg, P = 0.002; 2 studies, 129 participants). However, we are uncertain whether ketorolac has an important effect on opioid consumption, as the data were sparse and the results were inconsistent. Only one study reported data for opioid consumption when comparing ketorolac with an opioid. There were no clear differences between the ketorolac and opioid group at any time point. There were no data assessing this outcome for the comparison of ketorolac with another NSAID.There were insufficient data to allow us to analyze overall adverse event or serious adverse event rates. Although the majority of serious adverse events reported in those receiving ketorolac involved bleeding, the number of events was too low to conclude that bleeding risk was increased in those receiving ketorolac perioperatively. There was not a statistically significant increase in event rates for any specific adverse event, either in pooled analysis or in single studies, when comparing ketorolac and placebo. When comparing ketorolac with opioids or other NSAIDs, there were too few data to make any conclusions regarding event rates. Lastly, withdrawals due to adverse events were vary rare in all groups, reflecting the acute nature of such studies.We assessed the quality of evidence for all outcomes for each comparison (placebo or active) as very low, due to issues with risk of bias in individual studies, imprecision, heterogeneity between studies, and low overall numbers of participants and events.
Authors' conclusions: Due to the lack of data for our primary outcomes, and the very low-quality evidence for secondary outcomes, the efficacy and safety of ketorolac in treating postoperative pain in children were both uncertain. The evidence was insufficient to support or reject its use.
Conflict of interest statement
EM: none known. EM is a pharmacist with a Master's degree in Pain Research, Education and Policy, and manages patients with acute pain.
ER has received support from Pfizer for travel and accommodation (2015). ER is a pharmacist with a Master's degree in Pain Research, Education and Policy, and manages paediatric patients with acute pain.
TC: none known.
The protocol for this review was identified in a 2019 audit as not meeting the current definition of the Cochrane Commercial Sponsorship policy. At the time of its publication it was compliant with the interpretation of the existing policy. A new author team fully compliant with the 2014 policy completed the review. As with all reviews, new and updated, at update this review will be revised according to 2020 policy update.
Figures























Update of
References
References to studies included in this review
Chiaretti 1997 {published data only}
-
- Chiaretti A, Simeone E, Langer A, Butera G, Piastra M, Tortorolo L, et al. Comparison of ketorolac and fentanyl for pain relief in pediatric intensive care [Efficacia analgesica del ketorolac e del fentanyl in terapia intensiva pediatrica]. La Pediatria Medica e Chirugica 1997;19(6):419-24. - PubMed
Davis 1999 {published data only}
-
- Davis PJ, Greenberg JA, Gendelman M, Fertal K. Recovery characteristics of sevoflurane and halothane in preschool-aged children undergoing bilateral myringotomy and pressure equalization tube insertion. Anesthesia and Analgesia 1999;88(1):34-8. - PubMed
Gunter 1995 {published data only}
-
- Gunter JB, Varughese AM, Harrington JF, Wittkugel EP, Patankar SS, Matar MM, et al. Recovery and complications after tonsillectomy in children: a comparison of ketorolac and morphine. Anesthesia and Analgesia 1995;81(6):1136-41. - PubMed
Hamza 2012 {published data only}
-
- Hamza A, Hayat U, Khan Q, Khan M. To compare the efficacy of ketorolac and pethidine for postoperative pain relief in first 24 hours after tonsillectomy. Pakistan Journal of Medical and Health Sciences 2012;6(2):326-8.
Lieh‐Lai 1999 {published data only}
-
- Lieh-Lai MW, Kauffman RE, Uy HG, Danjin M, Simpson PM. A randomized comparison of ketorolac tromethamine and morphine for postoperative analgesia in critically ill children. Critical Care Medicine 1999;27(12):2786-91. - PubMed
Lynn 2007 {published data only}
-
- Lynn AM, Bradford H, Kantor ED, Seng KY, Salinger DH, Chen J, et al. Postoperative ketorolac tromethamine use in infants aged 6-18 months: the effect on morphine usage, safety assessment, and stereo-specific pharmacokinetics. Anesthesia and Analgesia 2007;104(5):1040-51. - PubMed
Maunuksela 1992 {published data only}
-
- Maunuksela EL, Kokki H, Bullingham RE. Comparison of intravenous ketorolac with morphine for postoperative pain in children. Clinical Pharmacology & Therapeutics 1992;52(4):436-43. - PubMed
Munoz‐Cuevas 1997 {published data only}
-
- Muñoz Cuevas H, Fernández L, Carmen M, Martínez Segura RT, Zavala de la Rosa JE, Zinher Espino E. Postoperative pain control in children postoperated of ophthalmic surgery [Control del dolor con Ketorolac en niños postoperados de cirugía oftalmológica]. Revista Mexicana de Anestesiologia 1997;20(4):180-3.
Munro 2002 {published data only}
-
- Munro HM, Walton SR, Malviya S, Merkel S, Voepel-Lewis T, Loder RT, et al. Low-dose ketorolac improves analgesia and reduces morphine requirements following posterior spinal fusion in adolescents. Canadian Journal of Anaesthesia 2002;49(5):461-6. - PubMed
Romsing 1998 {published data only}
-
- Romsing J, Ostergaard D, Walther-Larsen S, Valentin N. Analgesic efficacy and safety of preoperative versus postoperative ketorolac in paediatric tonsillectomy. Acta Anaesthesiologica Scandinavica 1998;42(7):770-5. - PubMed
Saryazdi 2016 {published data only}
-
- Saryazdi HH, Aghadavoudi OM, Shafa AM, Masoumi AM, Saberian PA. A comparative study of the analgesic effect of intravenous pethidine vs. ketorolac after inguinal hernia surgery in children under general anesthesia. Middle East Journal of Anaesthesiology 2016;23(5):527-33. - PubMed
Sutters 1995 {published data only}
-
- Sutters KA, Levine JD, Dibble S, Savedra M, Miaskowski C. Analgesic efficacy and safety of single-dose intramuscular ketorolac for postoperative pain management in children following tonsillectomy. Pain 1995;61(1):145-53. - PubMed
Sutters 1999 {published data only}
-
- Sutters KA, Shaw BA, Gerardi JA, Hebert D. Comparison of morphine patient-controlled analgesia with and without ketorolac for postoperative analgesia in pediatric orthopedic surgery. The American Journal of Orthopedics 1999;28(6):351-8. - PubMed
References to studies excluded from this review
Glickman 1995 {published data only}
-
- Glickman G, Olazabal A, Corcoran J. Comparative effects of Toradol® and Ibuprofen in the control of endodontic postoperative pain. Journal of Dental Research 1995;74(Suppl):27.
Greco 1994 {published data only}
-
- Greco R, Piastra M, Iacovacci V, Belcastro F, Forastiere AMS, Proietti S, et al. Continuous venous infusion of buprenorphine with autonomous elastomeric system in the control of postoperative pain. Minerva Anestesiologica 1994;60(Suppl 2):1-8. - PubMed
Gupta 2004 {published data only}
-
- Gupta A, Daggett C, Drant S, Rivero N, Lewis A. Prospective randomized trial of ketorolac after congenital heart surgery. Journal of Cardiothoracic and Vascular Anesthesia 2004;18(4):454-7. - PubMed
Hayes 2011 {published data only}
-
- Hayes JA, Forrest CR, Walsh W, Petroz GC, Adeli K, Bissonnette B. Continuous bupivacaine infusion post-iliac crest bone graft harvesting in pediatric cleft surgery: role and comparison with ketorolac. Cleft Palate-Craniofacial Journal 2011;48(5):532-7. - PubMed
Hernandez 1996 {published data only}
-
- Hernández Márquez VM, Toranzo Fernández JM, Guevara L, Javier F. A comparative study among ibuprofen, ketorolac and buprenorphine in postoperative pain control of third molar removal [Estudio comparativo entre ibuprofeno, buprenorfina y ketorolac en el control postoperatorio del dolor en la remoción de terceros molares]. Revista Asociación Dental Mexicana 1996;53(2):99-102.
Palacio 1997 {published data only}
-
- Palacio MA, Castejon J, Galvez R, Garcia-Sanchez MJ, Vazquez-Alonso E, Peran F, et al. Analgesia controlled by the patient with ketorolac in the treatment of postoperative pain in pediatric surgery [Analgesia controlada por el paciente con ketorolaco en el tratamiento del dolor postoperatorio en cirugia pediatrica]. Revista de la Sociedad Espanola del Dolor 1997;4(Suppl I):12-7.
Petrov 2009 {published data only}
-
- Petrov VI, Sabanov AV, Medvedev VG, Semenov PA, Tyrsin OI. Efficacy of lornoxicam and ketorolac in the prevention and treatment of postoperative pain syndrome in neurosurgical patients. Khirurgiia Moskva 2009;2:64-70. - PubMed
Rossitto 2009 {published data only}
-
- Rossitto M, Pante S, Manfre A, Ciccolo A. Post-operative analgesia in case of ano-rectal diseases. Annali Italiani di Chirurgia 2009;80(6):459-61. - PubMed
Vetter 1994 {published data only}
-
- Vetter TR, Heiner EJ. Intravenous ketorolac as an adjuvant to pediatric patient-controlled analgesia with morphine. Journal of Clinical Anethesia 1994;6(2):110-3. - PubMed
References to studies awaiting assessment
Tariq 2004 {published data only}
-
- Tariq GR, Mian MA, Chaudry IA. Comparison of intraperitoneal analgesia with bupivacaine/bupivacaine with ketorolac after laparoscopic cholecystectomy. Journal of Surgery Pakistan 2004;9(3):9-13.
References to ongoing studies
NCT01667120 {published data only}
-
- NCT01667120. The use of ketorolac in surgical neonates. clinicaltrials.gov/ct2/show/NCT01667120 (first received 15 August 2012).
NCT02653742 {published data only}
-
- NCT02653742. Ketorolac sublingual vs. fentanyl intranasal in pain control for bilateral myringotomy and tubes (BMT) placement in children. clinicaltrials.gov/ct2/show/NCT02653742 (first received 8 January 2016).
NCT02973958 {published data only}
-
- NCT02973958. Evaluating pain outcomes of ketorolac administration in children undergoing circumcision. clinicaltrials.gov/show/NCT02973958 (first received 18 November 2016).
NCT03178539 {published data only}
-
- NCT03178539. Comparative study between the effect of diclofanic and ketorolac in post tonsillectomy pain management. clinicaltrials.gov/ct2/show?id=NCT01363076 (first received 5 June 2017).
Additional references
APS 2008
-
- American Pain Society (APS). Management of acute pain and cancer pain with analgesics. In: Principles of analgesic use in the treatment of acute pain and cancer pain. 6th edition. Glenview, IL: American Pain Society, 2008.
Baley 2014
-
- Baley K, Michalov K, Kossick MA, McDowell M. Intravenous acetaminophen and intravenous ketorolac for management of pediatric surgical pain: a literature review. AANA Journal 2014;82(1):53-64. - PubMed
Bookstaver 2010
Brasher 2014
Chan 2014
Chou 2016
-
- Chou R, Gordon DB, Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. The Journal of Pain 2016;17(2):131-57. - PubMed
Cohen 2011
-
- Cohen MN, Christians U, Henthorn T, Vu Tran Z, Moll V, Zuk J, et al. Pharmacokinetics of single-dose intravenous ketorolac in infants aged 2-11 months. Anesthesia and Analgesia 2011;112(3):655-60. - PubMed
Cook 1995
Dechartres 2013
Dechartres 2014
-
- Dechartres A, Altman DG, Trinquart L, Boutron I, Ravaud P. Association between analytic strategy and estimates of treatment outcomes in meta-analyses. JAMA 2013;312:623-30. [DOI: ] - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses [Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011]. Available from handbook.cochrane.org.
Dsida 2002
eMC 2017
-
- UK Medicines and Healthcare Products Regulatory Agency (MHRA) and the European Medicines Agency (EMA). Ketorolac 30mg/ml solution for injection. In: Datapharm Communications Ltd. electronic Medicines Compendium (eMC 1999; updated 17 February 2017). www.medicines.org.uk/emc/medicine/20922 (accessed June 2018).
Forrest 1997
-
- Forrest JB, Heitlinger EL, Revell S. Ketorolac for postoperative pain management in children. Drug Safety 1997;16(5):309-29. - PubMed
GRADEpro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime, Inc.) GRADEpro GDT. Version (accessed June 26, 2018). Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2015.Available at gradepro.org.
Higgins 2011
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holdsworth 2003
Hurst 2004
-
- Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. Journal of Manipulative and Physiological Therapeutics 2004;27(1):26-35. - PubMed
Jacox 1994
-
- Jacox A, Carr DB, Payne R, Berde CB, Brietbart W, Cain JM, et al. Management of Cancer Pain. Clinical Practice Guideline Number 9. AHCPR Publication no. 94-0592. Rockville, MD: Agency for Health Care Policy and Research, 1994.
Jitpakdee 2014
Johnston 2012
Kaushal 2001
Kokki 2003
Kossowsky 2015
Lewis 2013
Lexicomp 2015 [Computer program]
-
- Wolters Kluwer Clinical Drug Information, Inc Ketorolac (systemic). In: Lexicomp Online, Pediatric and Neonatal Lexi-Drugs Online, Hudson, Ohio: Lexi-Comp, Inc. Wolters Kluwer Clinical Drug Information, Inc, (accessed June 26, 2018).
Mak 2011
McGrath 2008
-
- McGrath PH, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, et al. Core outcome domains and measures for acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. The Journal of Pain 2008;9(9):771-83. - PubMed
MHRA 2007
-
- Medicines and Healthcare Products Regulatory Agency. Ketoprofen and ketorolac: gastrointestinal risk. www.gov.uk/drug-safety-update/ketoprofen-and-ketorolac-gastrointestinal-... (accessed 24 January 2018).
Michelet 2012
-
- Michelet D, Andreu-Gallien J, Bensalah T, Hilly J, Wood C, Nivoche Y, et al. A meta-analysis of the use of nonsteroidal antiinflammatory drugs for pediatric postoperative pain. Anesthesia & Analgesia 2012;114(2):393-406. - PubMed
Moher 2009
Moore 1998
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA, editors(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15-24.
Nüesch 2010
Papacci 2004
-
- Papacci P, De Francisci G, Iacobucci T, Giannantonio C, De Carolis MP, Zecca E, et al. Use of intravenous ketorolac in the neonate and premature babies. Paediatric Anesthesia 2004;14(6):487-92. - PubMed
PaPaS 2012
-
- Cochrane Pain, Palliative and Supportive Care Review Group. PaPaS Author and Referee Guidance. papas.cochrane.org/papas-documents (accessed 6 November 2015).
Pasero 2011
-
- Pasero C, McCaffery M. Perioperative nonopioid use. In: Pain Assessment and Pharmacologic Management. St Louis, Missouri: Mosby, 2011.
Pharma Letter 1993
-
- Syntex' ketorolac withdrawn in Germany. In: The Pharma Letter 21 June 1993. www.thepharmaletter.com/article/syntex-ketorolac-withdrawn-in-germany (accessed 24 January 2018).
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riggin 2013
-
- Riggin L, Ramakrishna J, Sommer DD, Koren G. A 2013 updated systematic review & meta-analysis of 36 randomized controlled trials; no apparent effects of non steriodal anti-inflammatory agents on the risk of bleeding after tonsillectomy. Clinical Otolaryngology 2013;38(2):115-29. [DOI: 10.1111/coa.12106] - DOI - PubMed
Rohatgi 2016 [Computer program]
-
- WebPlotDigitizer. Version 3.10. Austin, Texas, USA: Ankit Rohatgi, May 2016.automeris.io/WebPlotDigitizer/.
Schultz‐Machata 2014
Somaini 2015
-
- Somaini M, Sahillioğlu E, Marzorati C, Lovisari F, Engelhardt T, Ingelmo PM. Emergence delirium, pain or both? A challenge for clinicians. Paediatric Anesthesia 2015;25(5):524-9. - PubMed
Standing 2009a
Standing 2009b
Stanko 2013
-
- Stanko D, Bergesio R, Davies K, Hegarty M, Ungern-Sternberg BS. Postoperative pain, nausea and vomiting following adeno-tonsillectomy - a long-term follow-up. Pediatric Anesthesia 2013;23(8):690-6. - PubMed
Takata 2008
Thorlund 2011
Tobias 2014
-
- Tobias JD. Acute pain management in infants and children - Part 2: Intravenous opioids, intravenous nonsteroidal anti-inflammatory drugs, and managing adverse effects. Pediatric Annals 2014;43(7):e169-75. - PubMed
Voepel‐Lewis 2008
-
- Voepel-Lewis T, Marinkovic A, Kostrzewa A, Tait A, Malviya S. The prevalence of an risk factors for adverse events in children receiving patient-controlled analgesia by proxy or patient-controlled analgesia after surgery. Anesthesia and Analgesia 2008;107(1):70-5. [DOI: 10.1213/ane.0b013e318172fa9e] - DOI - PubMed
Voepel‐Lewis 2012
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

