Omega-3 polyunsaturated fatty acids favourably modulate cardiometabolic biomarkers in type 2 diabetes: a meta-analysis and meta-regression of randomized controlled trials
- PMID: 29981570
- PMCID: PMC6035402
- DOI: 10.1186/s12933-018-0740-x
Omega-3 polyunsaturated fatty acids favourably modulate cardiometabolic biomarkers in type 2 diabetes: a meta-analysis and meta-regression of randomized controlled trials
Abstract
Background: Randomized controlled trials (RCTs) suggest that supplementation with omega-3 polyunsaturated fatty acids (n-3PUFAs) may favourably modify cardiometabolic biomarkers in type 2 diabetes (T2DM). Previous meta-analyses are limited by insufficient sample sizes and omission of meta-regression techniques, and a large number of RCTs have subsequently been published since the last comprehensive meta-analysis. Updated information regarding the impact of dosage, duration or an interaction between these two factors is therefore warranted. The objective was to comprehensively assess the effect of n-3PUFAs supplementation on cardiometabolic biomarkers including lipid profiles, inflammatory parameters, blood pressure, and indices of glycaemic control, in people with T2DM, and identify whether treatment dosage, duration or an interaction thereof modify these effects.
Methods: Databases including PubMed and MEDLINE were searched until 13th July 2017 for RCTs investigating the effect of n-3PUFAs supplementation on lipid profiles, inflammatory parameters, blood pressure, and indices of glycaemic control. Data were pooled using random-effects meta-analysis and presented as standardised mean difference (Hedges g) with 95% confidence intervals (95% CI). Meta-regression analysis was performed to investigate the effects of duration of supplementation and total dosage of n-3PUFAs as moderator variables where appropriate.
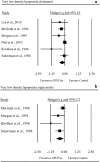
Results: A total of 45 RCTs were identified, involving 2674 people with T2DM. n-3PUFAs supplementation was associated with significant reductions in LDL [ES: - 0.10, (95% CI - 0.17, - 0.03); p = 0.007], VLDL (ES: - 0.26 (- 0.51, - 0.01); p = 0.044], triglycerides (ES: - 0.39 (- 0.55, - 0.24; p ≤ 0.001] and HbA1c (ES: - 0.27 (- 0.48, - 0.06); p = 0.010]. Moreover, n-3PUFAs supplementation was associated with reduction in plasma levels of TNF-α [ES: - 0.59 (- 1.17, - 0.01); p = 0.045] and IL-6 (ES: - 1.67 (- 3.14, - 0.20); p = 0.026]. All other lipid markers, indices of glycaemic control, inflammatory parameters, and blood pressure remained unchanged (p > 0.05).
Conclusions: n-3PUFAs supplementation produces favourable hypolipidemic effects, a reduction in pro-inflammatory cytokine levels and improvement in glycaemia. Neither duration nor dosage appear to explain the observed heterogeneity in response to n-3PUFAs. Trial registration This trial was registered at http://www.crd.york.ac.uk as CRD42016050802.
Keywords: Cardiovascular disease; Docosahexaenoic acid; Eicosapentaenoic acid; Meta-analysis; Omega-3 polyunsaturated fatty acids; Type 2 diabetes.
Figures





References
-
- Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

