Are compound leaves more complex than simple ones? A multi-scale analysis
- PMID: 29982438
- PMCID: PMC6324747
- DOI: 10.1093/aob/mcy116
Are compound leaves more complex than simple ones? A multi-scale analysis
Abstract
Background and aims: The question of which cellular mechanisms determine the variation in leaf size has been addressed mainly in plants with simple leaves. It is addressed here in tomato taking into consideration the expected complexity added by the several lateral appendages making up the compound leaf, the leaflets.
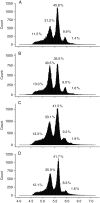
Methods: Leaf and leaflet areas, epidermal cell number and areas, and endoreduplication (co-) variations were analysed in Solanum lycopersicum considering heteroblastic series in a wild type (Wva106) and an antisense mutant, the Pro35S:Slccs52AAS line, and upon drought treatments. All plants were grown in an automated phenotyping platform, PHENOPSIS, adapted to host plants grown in 7 L pots.
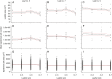
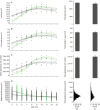
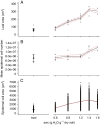
Key results: Leaf area, leaflet area and cell number increased with leaf rank until reaching a plateau. In contrast, cell area slightly decreased and endoreduplication did not follow any trend. In the transgenic line, leaf area, leaflet areas and cell number of basal leaves were lower than in the wild type, but higher in upper leaves. Reciprocally, cell area was higher in basal leaves and lower in upper leaves. When scaled up at the whole sympodial unit, all these traits did not differ significantly between the transgenic line and the wild type. In response to drought, leaf area was reduced, with a clear dose effect that was also reported for all size-related traits, including endoreduplication.
Conclusions: These results provide evidence that all leaflets have the same cellular phenotypes as the leaf they belong to. Consistent with results reported for simple leaves, they show that cell number rather than cell size determines the final leaf areas and that endoreduplication can be uncoupled from leaf and cell sizes. Finally, they re-question a whole-plant control of cell division and expansion in leaves when the Wva106 and the Pro35S:Slccs52AAS lines are compared.
Figures




References
-
- Ashby E. 1948. Studies in the morphogenesis of leaves. 2. The area, cell size and cell number of leaves of Ipomoea in relation to their position on the shoot. New Phytologist 47: 177–195.
-
- Breuer C, Ishida T, Sugimoto K. 2010. Developmental control of endocycles and cell growth in plants. Current Opinion in Plant Biology 13: 654–660. - PubMed
-
- Champagne C, Sinha N. 2004. Compound leaves, equal to the sum of their parts?Development 131: 4401–4412. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

