Pharmacological and physiological activation of AMPK improves the spliceopathy in DM1 mouse muscles
- PMID: 29982462
- PMCID: PMC6140770
- DOI: 10.1093/hmg/ddy245
Pharmacological and physiological activation of AMPK improves the spliceopathy in DM1 mouse muscles
Abstract
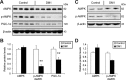
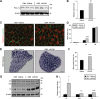
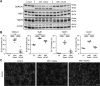
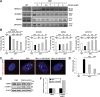
Myotonic dystrophy type 1 (DM1) is a debilitating multisystemic disorder caused by a triplet repeat expansion in the 3' untranslated region of dystrophia myotonica protein kinase mRNAs. Mutant mRNAs accumulate in the nucleus of affected cells and misregulate RNA-binding proteins, thereby promoting characteristic missplicing events. However, little is known about the signaling pathways that may be affected in DM1. Here, we investigated the status of activated protein kinase (AMPK) signaling in DM1 skeletal muscle and found that the AMPK pathway is markedly repressed in a DM1 mouse model (human skeletal actin-long repeat, HSALR) and patient-derived DM1 myoblasts. Chronic pharmacological activation of AMPK signaling in DM1 mice with 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR) has multiple beneficial effects on the DM1 phenotype. Indeed, a 6-week AICAR treatment of DM1 mice promoted expression of a slower, more oxidative phenotype, improved muscle histology and corrected several events associated with RNA toxicity. Importantly, AICAR also had a dose-dependent positive effect on the spliceopathy in patient-derived DM1 myoblasts. In separate experiments, we also show that chronic treatment of DM1 mice with resveratrol as well as voluntary wheel running also rescued missplicing events in muscle. Collectively, our findings demonstrate the therapeutic potential of chronic AMPK stimulation both physiologically and pharmacologically for DM1 patients.
Figures




References
-
- Fu Y.H., Pizzuti A., Fenwick R.G. Jr., King J., Rajnarayan S., Dunne P.W., Dubel J., Nasser G.A., Ashizawa T., Jong P. et al. (1992) An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science, 255, 1256–1258. - PubMed
-
- Tsilfidis C., MacKenzie A.E., Mettler G., Barcelo J. and Korneluk R.G. (1992) Correlation between CTG trinucleotide repeat length and frequency of severe congenital myotonic dystrophy. Nat. Genet., 1, 192–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

