Definition of hidden drug cardiotoxicity: paradigm change in cardiac safety testing and its clinical implications
- PMID: 29982507
- PMCID: PMC6554653
- DOI: 10.1093/eurheartj/ehy365
Definition of hidden drug cardiotoxicity: paradigm change in cardiac safety testing and its clinical implications
Abstract
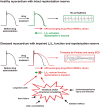
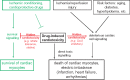
Unexpected cardiac adverse effects are the leading causes of discontinuation of clinical trials and withdrawal of drugs from the market. Since the original observations in the mid-90s, it has been well established that cardiovascular risk factors and comorbidities (such as ageing, hyperlipidaemia, and diabetes) and their medications (e.g. nitrate tolerance, adenosine triphosphate-dependent potassium inhibitor antidiabetic drugs, statins, etc.) may interfere with cardiac ischaemic tolerance and endogenous cardioprotective signalling pathways. Indeed drugs may exert unwanted effects on the diseased and treated heart that is hidden in the healthy myocardium. Hidden cardiotoxic effects may be due to (i) drug-induced enhancement of deleterious signalling due to ischaemia/reperfusion injury and/or the presence of risk factors and/or (ii) inhibition of cardioprotective survival signalling pathways, both of which may lead to ischaemia-related cell death and/or pro-arrhythmic effects. This led to a novel concept of 'hidden cardiotoxicity', defined as cardiotoxity of a drug that manifests only in the diseased heart with e.g. ischaemia/reperfusion injury and/or in the presence of its major comorbidities. Little is known on the mechanism of hidden cardiotoxocity, moreover, hidden cardiotoxicity cannot be revealed by the routinely used non-clinical cardiac safety testing methods on healthy animals or tissues. Therefore, here, we emphasize the need for development of novel cardiac safety testing platform involving combined experimental models of cardiac diseases (especially myocardial ischaemia/reperfusion and ischaemic conditioning) in the presence and absence of major cardiovascular comorbidities and/or cotreatments.
Keywords: Cardiac; Comedication; Comorbidity; Conditioning; Heart; Ischaemia; Post-conditioning; Pre-conditioning; Remote conditioning; Safety; Toxicity.
© The Author(s) 2018. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

References
-
- Onakpoya IJ, Heneghan CJ, Aronson JK.. Worldwide withdrawal of medicinal products because of adverse drug reactions: a systematic review and analysis. Crit Rev Toxicol 2016;46:477–489. - PubMed
-
- Onakpoya IJ, Heneghan CJ, Aronson JK.. Post-marketing withdrawal of analgesic medications because of adverse drug reactions: a systematic review. Expert Opin Drug Saf 2018;17:63–72. - PubMed
-
- Faria J, Barbosa J, Leal S, Afonso LP, Lobo J, Moreira R, Queiros O, Carvalho F, Dinis-Oliveira RJ.. Effective analgesic doses of tramadol or tapentadol induce brain, lung and heart toxicity in Wistar rats. Toxicology 2017;385:38–47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

