Boosting of Mucosal Immunity After Fractional-Dose Inactivated Poliovirus Vaccine
- PMID: 29982532
- PMCID: PMC9161111
- DOI: 10.1093/infdis/jiy389
Boosting of Mucosal Immunity After Fractional-Dose Inactivated Poliovirus Vaccine
Abstract
Background: Inactivated poliovirus vaccine (IPV) boosts mucosal immunity in persons previously vaccinated with oral poliovirus vaccine (OPV). We assessed whether fractional-dose IPV (fIPV, 1/5th of full dose) administered intradermally also boosts mucosal immunity.
Methods: Children 10-12 years old were enrolled in Sri Lanka and randomized to receive one dose IPV, fIPV, or no IPV vaccine. One month later, they received OPV challenge. Blood was collected at enrolment and before challenge; stool was collected at 3, 7, and 14 days post-challenge. Sera were analysed for presence of poliovirus neutralizing antibodies; stool was analysed for poliovirus.
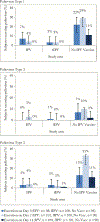
Results: We analysed 304/309 (98%) enrolled subjects. There were 16/97 (16%), 9/99 (9%), and 72/95 (76%) subjects excreting poliovirus after challenge in the IPV, fIPV and "No IPV Vaccine" study arms, respectively (P < .001 for comparison of IPV [or fIPV] vs "No IPV Vaccine"; P = .1 for comparisons of fIPV vs IPV). Relative decrease in excretion prevalence was 80% and 88% to IPV and fIPV, respectively, compared with the "No IPV Vaccine" control arm.
Conclusions: Single fIPV dose boosted mucosal immunity to a similar degree as single full dose of IPV. This finding provides further evidence in support of fIPV for poliovirus outbreak response at the time of IPV global supply shortage.
Clinical trials registration: Australia New Zealand Clinical Trial Registry ACTRN12616000124437p.
Conflict of interest statement
Figures


Comment in
-
Role of Fractional-Dose Intradermal Inactivated Poliovirus Vaccine in Halting Polio Transmission: Finding the Missing Piece for Global Polio Eradication.J Infect Dis. 2018 Nov 5;218(12):1855-1857. doi: 10.1093/infdis/jiy390. J Infect Dis. 2018. PMID: 29982585 No abstract available.
References
-
- World Health Organization. Wild poliovirus list. http://www.polioeradication.org/Dataandmonitoring/Poliothisweek/Wildpoli.... Accessed 12 February 2018.
-
- Centers for Disease Control and Prevention. Apparent global interruption of wild poliovirus type 2 transmission. MMWR Morb Mortal Wkly Rep 2001; 50:222–4. - PubMed
-
- World Health Organization. Immunization, Vaccines and Biologicals. Data, statistics and graphics. http://www.who.int/immunization/monitoring_surveillance/data/en/. Accessed 10 July 2017.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

