Effects of Treatment Delay on Efficacy of Tecovirimat Following Lethal Aerosol Monkeypox Virus Challenge in Cynomolgus Macaques
- PMID: 29982575
- PMCID: PMC6151088
- DOI: 10.1093/infdis/jiy326
Effects of Treatment Delay on Efficacy of Tecovirimat Following Lethal Aerosol Monkeypox Virus Challenge in Cynomolgus Macaques
Abstract
Background: Tecovirimat (ST-246) is being developed as an antiviral therapeutic for smallpox for use in the event of an accidental or intentional release. The last reported case of smallpox was 1978 but the potential for use of variola virus for biowarfare has renewed interest in smallpox antiviral therapeutics.
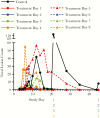
Methods: Cynomolgus macaques were challenged with a lethal dose of monkeypox virus (MPXV) by aerosol as a model for human smallpox and treated orally with 10 mg/kg tecovirimat once daily starting up to 8 days following challenge. Monkeys were monitored for survival, lesions, and clinical signs of disease. Samples were collected for measurement of viremia by quantitative real-time polymerase chain reaction, and for white blood cell counts.
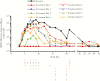
Results: Survival in animals initiating treatment up to 5 days postchallenge was 100%. In animals treated starting 6, 7, or 8 days following challenge, survival was 67%, 100%, and 50%, respectively. Treatment initiation up to 4 days following challenge reduced severity of clinical manifestations of infection.
Conclusions: Tecovirimat treatment initiated up to 8 days following a lethal aerosol MPXV challenge improves survival and, when initiated earlier than 5 days after challenge, provides protection from clinical effects of disease, supporting the conclusion that it is a promising smallpox antiviral therapeutic candidate.
Figures



References
-
- Barclay WR. The conquest of smallpox. JAMA 1978; 240:1991–2. - PubMed
-
- World Health Organization. The global eradication of smallpox: final report of the Global Commission for the Certification of Smallpox Eradication, Geneva, December 1979. Geneva, Switzerland: WHO, 1980.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

