Spatiotemporal heterogeneity of tumor vasculature during tumor growth and antiangiogenic treatment: MRI assessment using permeability and blood volume parameters
- PMID: 29983002
- PMCID: PMC6089152
- DOI: 10.1002/cam4.1624
Spatiotemporal heterogeneity of tumor vasculature during tumor growth and antiangiogenic treatment: MRI assessment using permeability and blood volume parameters
Abstract
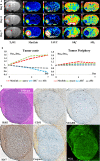
Tumor heterogeneity is an important concept when assessing intratumoral variety in vascular phenotypes and responses to antiangiogenic treatment. This study explored spatiotemporal heterogeneity of vascular alterations in C6 glioma mice during tumor growth and antiangiogenic treatment on serial MR examinations (days 0, 4, and 7 from initiation of vehicle or multireceptor tyrosine kinase inhibitor administration). Transvascular permeability (TP) was quantified on dynamic-contrast-enhanced MRI (DCE-MRI) using extravascular extracellular agent (Gd-DOTA); blood volume (BV) was estimated using intravascular T2 agent (SPION). With regard to region-dependent variability in vascular phenotypes, the control group demonstrated higher TP in the tumor center than in the periphery, and greater BV in the tumor periphery than in the center. This distribution pattern became more apparent with tumor growth. Antiangiogenic treatment effect was regionally heterogeneous: in the tumor center, treatment significantly suppressed the increase in TP and decrease in BV (ie, typical temporal change in the control group); in the tumor periphery, treatment-induced vascular alterations were insignificant and BV remained high. On histopathological examination, the control group showed greater CD31, VEGFR2, Ki67, and NG2 expression in the tumor periphery than in the center. After treatment, CD31 and Ki67 expression was significantly suppressed only in the tumor center, whereas VEGFR2 and α-caspase 3 expression was decreased and NG2 expression was increased in the entire tumor. These results demonstrate that MRI can reliably depict spatial heterogeneity in tumor vascular phenotypes and antiangiogenic treatment effects. Preserved angiogenic activity (high BV on MRI and high CD31) and proliferation (high Ki67) in the tumor periphery after treatment may provide insights into the mechanism of tumor resistance to antiangiogenic treatment.
Keywords: magnetic resonance imaging; spatial heterogeneity; tumor vessels.
© 2018 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures





References
-
- Marusyk A, Almendro V, Polyak K. Intra‐tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12:323‐334. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

