Electric Fields Elicit Ballooning in Spiders
- PMID: 29983315
- PMCID: PMC6065530
- DOI: 10.1016/j.cub.2018.05.057
Electric Fields Elicit Ballooning in Spiders
Abstract
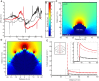
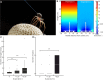
When one thinks of airborne organisms, spiders do not usually come to mind. However, these wingless arthropods have been found 4 km up in the sky [1], dispersing hundreds of kilometers [2]. To disperse, spiders "balloon," whereby they climb to the top of a prominence, let out silk, and float away. The prevailing view is that drag forces from light wind allow spiders to become airborne [3], yet ballooning mechanisms are not fully explained by current aerodynamic models [4, 5]. The global atmospheric electric circuit and the resulting atmospheric potential gradient (APG) [6] provide an additional force that has been proposed to explain ballooning [7]. Here, we test the hypothesis that electric fields (e-fields) commensurate with the APG can be detected by spiders and are sufficient to stimulate ballooning. We find that the presence of a vertical e-field elicits ballooning behavior and takeoff in spiders. We also investigate the mechanical response of putative sensory receivers in response to both e-field and air-flow stimuli, showing that spider mechanosensory hairs are mechanically activated by weak e-fields. Altogether, the evidence gathered reveals an electric driving force that is sufficient for ballooning. These results also suggest that the APG, as additional meteorological information, can reveal the auspicious time to engage in ballooning. We propose that atmospheric electricity adds key information to our understanding and predictive capability of the ecologically important mass migration patterns of arthropod fauna [8]. VIDEO ABSTRACT.
Keywords: atmospheric potential gradient; ballooning; electrostatics; mechanoreception; sensory ecology; spider; trichobothria.
Copyright © 2018 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Glick, P.A. (1939). The distribution of insects, spiders, and mites in the air. United States Department of Agriculture, technical bulletin no. 673. https://naldc.nal.usda.gov/download/CAT86200667/PDF.
-
- Bell J.R., Bohan D.A., Shaw E.M., Weyman G.S. Ballooning dispersal using silk: world fauna, phylogenies, genetics and models. Bull. Entomol. Res. 2005;95:69–114. - PubMed
-
- Weyman G.S. A review of the possible causative factors and significance of ballooning in spiders. Ethol. Ecol. Evol. 1993;5:279–291.
-
- Humphrey J.A.C. Fluid mechanic constraints on spider ballooning. Oecologia. 1987;73:469–477. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

