A retrospective study of concurrent chemoradiotherapy plus S-1 adjuvant chemotherapy on curative effect for treatment of patients with N3 stage nasopharyngeal carcinoma
- PMID: 29983590
- PMCID: PMC6025766
- DOI: 10.2147/CMAR.S165804
A retrospective study of concurrent chemoradiotherapy plus S-1 adjuvant chemotherapy on curative effect for treatment of patients with N3 stage nasopharyngeal carcinoma
Abstract
Introduction: The purpose of this study was to analyze the efficacy and safety of concurrent chemoradiotherapy plus S-1 adjuvant chemotherapy for N3 stage nasopharyngeal carcinoma (NPC).
Methods: This study included 44 N3 stage NPC patients treated with concurrent chemoradiotherapy plus S-1 adjuvant chemotherapy. The intensity-modulated radiation therapy doses were planning target volume (PTV) 70-72 Gy for gross disease in the nasopharynx and 66-70 Gy for positive lymph nodes. The doses for high-risk- and low-risk region PTV were 60-62 and 54-56 Gy in 31-33 fractions. All patients received a concurrent chemotherapy program consisting of cisplatin 100 mg/m2, day 1, and the cycle repetition was every 21 days. The adjuvant chemotherapy program consisted of 4 cycles of S-1. The dose of S-1 was determined according to the body surface area (BSA): 40 mg twice a day for BSA <1.25 m2; 50 mg twice a day for 1.25 m2≤BSA<1.5 m2; and 60 mg twice a day for BSA ≥1.5 m2. S-1 was given on days 1-28, given 6 weeks apart.
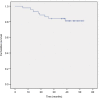
Results: All 44 patients completed at least 2 cycles of concurrent chemotherapy and 4 cycles of adjuvant chemotherapy. The total efficiency of therapy was 100.0%. The 3-year overall survival (OS), distant metastasis-free survival (DMFS), local-regional control, and progression-free survival rates were 86.4%, 84.1%, 97.7%, and 81.8%, respectively. There were no differences in the OS, DMFS, and efficiency between fast-fading group (reaching partial response before the second cycle of concurrent chemotherapy) and general-fading group (the rest of the group). The incidence of rash in the entire group was low, and there was also no association with prognosis.
Conclusion: In patients with N3 stage NPC, concurrent chemoradiotherapy plus S-1 adjuvant chemotherapy yielded an excellent survival benefit, and the toxicities were mild and tolerable. Distant metastasis was the main cause of treatment failure.
Keywords: S-1; chemotherapy; intensity-modulated radiotherapy; nasopharyngeal carcinoma; prognosis.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


References
-
- Liang X, Yang J, Gao T, et al. Nasopharynx Cancer Epidemiology in China. China Cancer. 2016;25(11):835–840. Chinese.
-
- Zong J, Lin S, Lin J, et al. Impact of intensity-modulated radiotherapy on nasopharyngeal carcinoma: validation of the 7th edition AJCC staging system. Oral Oncol. 2015;51(3):254–259. - PubMed
-
- Hsu C, Lee SH, Ejadi S, et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J Clin Oncol. 2017;35(36):4050–4056. - PubMed
-
- Strati A, Koutsodontis G, Papaxoinis G, et al. Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinoma. Ann Oncol. 2017;28(8):1923–1933. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

