Antimelanogenesis and skin-protective activities of Panax ginseng calyx ethanol extract
- PMID: 29983620
- PMCID: PMC6026384
- DOI: 10.1016/j.jgr.2018.02.007
Antimelanogenesis and skin-protective activities of Panax ginseng calyx ethanol extract
Abstract
Background: The antioxidant effects of Panax ginseng have been reported in several articles; however, little is known about the antimelanogenesis effect, skin-protective effect, and cellular mechanism of Panax ginseng, especially of P. ginseng calyx. To understand how an ethanol extract of P. ginseng berry calyx (Pg-C-EE) exerts skin-protective effects, we studied its activities in activated melanocytes and reactive oxygen species (ROS)-induced keratinocytes.
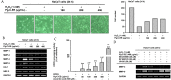
Methods: To confirm the antimelanogenesis effect of Pg-C-EE, we analyzed melanin synthesis and secretion and messenger RNA and protein expression levels of related genes. Ultraviolet B (UVB) and hydrogen peroxide (H2O2) were used to induce cell damage by ROS generation. To examine whether this damage is inhibited by Pg-C-EE, we performed cell viability assays and gene expression and transcriptional activation analyses.
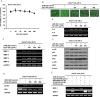
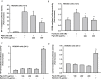
Results: Pg-C-EE inhibited melanin synthesis and secretion by blocking activator protein 1 regulatory enzymes such as p38, extracellular signal-regulated kinases (ERKs), and cyclic adenosine monophosphate response element-binding protein. Pg-C-EE also suppressed ROS generation induced by H2O2 and UVB. Treatment with Pg-C-EE decreased the expression of matrix metalloproteinases, mitogen-activated protein kinases, and hyaluronidases and increased the cell survival rate.
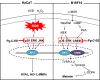
Conclusion: These results suggest that Pg-C-EE may have antimelanogenesis properties and skin-protective properties through regulation of activator protein 1 and cyclic adenosine monophosphate response element-binding protein signaling. Pg-C-EE may be used as a skin-improving agent, with moisture retention and whitening effects.
Keywords: Antimelanogenesis; Calyx of berry; Matrix metalloproteinases; Panax ginseng; Skin protective.
Figures



References
-
- Murphy K., Weaver C. Janeway's immunobiology. Garland Science. 2016
-
- Wilkinson P., Millington R. Cambridge university press; Cambridge (GB)[etc.]: 1983. Skin (Digitally printed version ed.)
-
- Kumar C.M., Sathisha U., Dharmesh S., Rao A.A., Singh S.A. Interaction of sesamol (3, 4-methylenedioxyphenol) with tyrosinase and its effect on melanin synthesis. Biochimie. 2011;93:562–569. - PubMed
-
- Laskin J.D., Piccinini L., Engelhardt D.L., Weinstein I.B. Control of melanin synthesis and secretion by B16/C3 melanoma cells. J Cell Physiol. 1982;113:481–486. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

