Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets
- PMID: 29984112
- PMCID: PMC6032665
- DOI: 10.1089/wound.2017.0761
Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets
Abstract
Significance: The immune system plays a central role in orchestrating the tissue healing process. Hence, controlling the immune system to promote tissue repair and regeneration is an attractive approach when designing regenerative strategies. This review discusses the pathophysiology of both acute and chronic wounds and possible strategies to control the immune system to accelerate chronic wound closure and promote skin regeneration (scar-less healing) of acute wounds. Recent Advances: Recent studies have revealed the key roles of various immune cells and immune mediators in skin repair. Thus, immune components have been targeted to promote chronic wound repair or skin regeneration and several growth factors, cytokines, and biomaterials have shown promising results in animal models. However, these novel strategies are often struggling to meet efficacy standards in clinical trials, partly due to inadequate drug delivery systems and safety concerns. Critical Issues: Excess inflammation is a major culprit in the dysregulation of normal wound healing, and further limiting inflammation effectively reduces scarring. However, current knowledge is insufficient to efficiently control inflammation and specific immune cells. This is further complicated by inadequate drug delivery methods. Future Directions: Improving our understanding of the molecular pathways through which the immune system controls the wound healing process could facilitate the design of novel regenerative therapies. Additionally, better delivery systems may make current and future therapies more effective. To promote the entry of current regenerative strategies into clinical trials, more evidence on their safety, efficacy, and cost-effectiveness is also needed.
Keywords: biomaterials; chronic wounds; immune system; immunomodulation; scarring; therapeutics.
Figures

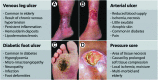
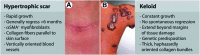
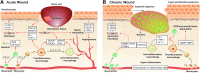
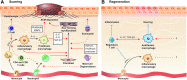
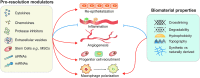
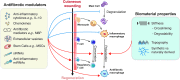
References
-
- Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008;372:139–144 - PubMed
-
- Julier Z, Park AJ, Briquez PS, Martino MM. Promoting tissue regeneration by modulating the immune system. Acta Biomater 2017;53:13–28 - PubMed
-
- Ramos-Torrecillas J, Garcia-Martinez O, De Luna-Bertos E, Ocana-Peinado FM, Ruiz C. Effectiveness of platelet-rich plasma and hyaluronic acid for the treatment and care of pressure ulcers. Biol Res Nurs 2015;17:152–158 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

