Inspired by Nature: Hydrogels as Versatile Tools for Vascular Engineering
- PMID: 29984113
- PMCID: PMC6032659
- DOI: 10.1089/wound.2017.0760
Inspired by Nature: Hydrogels as Versatile Tools for Vascular Engineering
Abstract
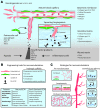
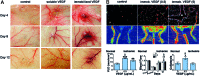
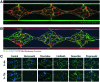
Significance: Diseases related to vascular malfunction, hyper-vascularization, or lack of vascularization are among the leading causes of morbidity and mortality. Engineered, vascularized tissues as well as angiogenic growth factor-releasing hydrogels could replace defective tissues. Further, treatments and testing of novel vascular therapeutics will benefit significantly from models that allow for the study of vascularized tissues under physiological relevant in vitro conditions. Recent Advances: Inspired by fibrin, the provisional matrix during wound healing, naturally derived and synthetic hydrogel scaffolds have been developed for vascular engineering. Today, engineers and biologists use commercially available hydrogels to pre-vascularize tissues, to control the delivery of angiogenic growth factors, and to establish vascular diseases models. Critical Issue: For clinical translation, pre-vascularized tissue constructs must be sufficiently large and stable to substitute function-relevant tissue defects and integrate with host vascular perfusion. Moreover, the continuous integration of knowhow from basic vascular biology with innovative, tailorable materials and advanced manufacturing technologies is key to achieving near-physiological tissue models and new treatments to control vascularization. Future Directions: For transplantation, engineered tissues must comprise hierarchically organized vascular trees of different caliber and function. The development of novel vascularization-promoting or -inhibiting therapeutics will benefit from physiologically relevant vessel models. In addition, tissue models representing treatment-relevant vascular tissue functions will increase the capacity to screen for therapeutic compounds and will significantly reduce the need for animals for their validation.
Keywords: angiogenesis; blood vessel; engineering; growth factor; hydrogel; tissue model.
Figures



References
-
- Potente M, Makinen T. Vascular heterogeneity and specialization in development and disease. Nat Rev Mol Cell Biol 2017;18:477–494 - PubMed
-
- Asahara T, Masuda H, Takahashi T, et al. . Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999;85:221–228 - PubMed
-
- Drake CJ. Embryonic and adult vasculogenesis. Birth Defects Res C Embryo Today 2003;69:73–82 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

