Hypometabolism of the posterior cingulate cortex is not restricted to Alzheimer's disease
- PMID: 29984170
- PMCID: PMC6030576
- DOI: 10.1016/j.nicl.2018.05.024
Hypometabolism of the posterior cingulate cortex is not restricted to Alzheimer's disease
Abstract
When differential diagnosis of dementia includes both Alzheimer's disease (AD) and the behavioural variant of frontotemporal dementia (bvFTD), distribution of cerebral glucose metabolism as measured using [18F]‑2‑fluoro‑2‑deoxy‑d‑glucose positron emission tomography ([18F]FDG-PET) may be helpful. One important clue for differentiation is the presence of hypometabolism in the posterior cingulate cortex (PCC), usually associated with AD. PCC hypometabolism however, could also be present in bvFTD. Therefore, the specificity of PCC hypometabolism was examined. Based on visual reading PCC hypometabolism was present in 69-73/81 probable AD patients, in 10-16/33 probable bvFTD patients, and in 0-1/22 cognitive normal (CN) subjects. Findings were validated using a PCC to reference tissue [18F]FDG standard uptake value ratio (SUVr) cut-off, which was derived from the receiver operating characteristic (ROC) separating probable AD from CN, resulting in 9-14/33 bvFTD patients having PCC hypometabolism, depending on the reference tissue used. In conclusion, PCC hypometabolism is not restricted to AD.
Keywords: Alzheimer's disease; Frontotemporal dementia; Hypometabolism; Posterior cingulate cortex; [18F]FDG-PET.
Figures
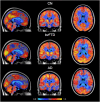

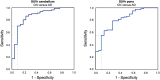


References
-
- Bastin C., Feyers D., Souchay C., Guillaume B., Pepin J.-L., Lemaire C., Degueldre C., Collette F., Salmon E. Frontal and posterior cingulate metabolic impairment in the behavioral variant of frontotemporal dementia with impaired autonoetic consciousness. Hum. Brain Mapp. 2012;33:1268–1278. - PMC - PubMed
-
- Broe M., Hodges J.R., Schofield E., Shepherd C.E., Kril J.J., Halliday G.M. Staging disease severity in pathologically confirmed cases of frontotemporal dementia. Neurology. 2003;60:1005–1011. - PubMed
-
- Buckner R.L., Snyder A.Z., Shannon B.J., Larossa G., Sachs R., Fotenos A.F., Sheline Y.I., Klunk W.E., Mathis C.A., Morris J.C., Mintun M.A. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J. Neurosci. 2005;25:7709–7717. - PMC - PubMed
-
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. - PubMed
-
- Diehl J., Grimmer T., Drzezga A., Riemenschneider M., Förstl H., Kurz A. Cerebral metabolic patterns at early stages of frontotemporal dementia and semantic dementia. A PET study. Neurobiol. Aging. 2004;25:1051–1056. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

